Abstract
Lymphoma is the neoplasia most commonly found in cats, with different forms of presentation of the disease. Among the various types of lymphoma, alimentary lymphoma is considered the most common in the oncology clinic in this species, reaching 50% of all diagnosed lymphomas. In general, this disease can be lymphocytic or lymphoblastic, differing as to the acute or chronic character of its manifestation. The B-cell lymphoblastic lymphoma seems to be the most severe and challenging to treat. The therapy of choice for this disease is conventional chemotherapy. However, this type of protocol results in a certain toxicity degree, often making the treatment sequence unfeasible. Within this context, complementary therapies stand out for not presenting side effects. Therefore, the ultra-diluted Viscum album becomes an excellent alternative for this therapeutic approach. This study aimed to report a case of feline alimentary lymphoma of B-cell lymphoblast type, treated by injectable, homeopathic therapy. A complete resolution of the mass manifestation was observed as well as an increase in the patient’s survival, differently from those cases described in the literature.
Introduction
Hematopoietic tumors are considered to have the highest incidence among domestic cats [1-5], comprising onethird of all tumors in cats. They can be systemic or multicentric and affect different tissues [5]. Viruses such as the feline leukemia virus (FeLV) [6,7] and the feline immunodeficiency virus (FIV) [8] are among the possible causes for this disease emergence. Only 25% of cats with lymphoma are positive for FeLV, and about 20% of cats that develop lymphoma in the United States are seropositive for antibodies against FIV [8,9]. There is no evidence for racial or sex predisposition. However, some authors claim that neutered males are slightly more affected by lymphoma, especially the alimentary type [3]. The average age of patients diagnosed with lymphoma is 8 to 10 years [2,10]. In general, the alimentary lymphoma type occurs in cats of about 10 to 12 years old, negative for FeLV [9]. It is the most diagnosed type among the different lymphoma types, comprising about 50% of the cases [11]. In this context, alimentary lymphoma is defined as lymphoid neoplasia that affects the gastrointestinal tract and regional lymph nodes, usually affecting the small intestine, liver, and spleen [9]. It is considered the second most common neoplasia found in the gastrointestinal tract of cats [12]. It affects 50% of the felines diagnosed with lymphoma [5]. This type of neoplasia may manifest itself in two ways: lymphocytic, also called small cells or poorlydifferentiated, and lymphoblastic, also called large cells or highly differentiated, which may extend beyond the gastrointestinal tract, reaching peripheral, thoracic, and bone marrow lymph nodes [13].
Lymphoblastic lymphoma is often related to more severe and acute symptoms adding to the rapid onset and progression. Nearly 80% of cats with this neoplasia will have some palpable abnormality in the abdomen, which may be mass, thickening of the intestinal loops accompanied by hiatus or splenomegaly [14]. This neoplasia usually starts in the digestive system of cats and quickly spreads to other organs and systems. It rarely has a significant response to treatment [14]. The most prevalent immunophenotype in lymphocytic lymphomas is T cells, whereas the B cells are more likely to be in the lymphoblastic [15]. Affected cats generally have anorexia, significant weight loss, and a higher probability of presenting intussusception, obstructive masses, and septic peritonitis, resulting in a perforation. Cats with lymphoblastic lymphoma may or may not have a vomiting and diarrhea history [3].
The alimentary lymphoma diagnosis is performed by clinical history, physical examination, laboratory and imaging tests, aspiration cytology, biopsy, and immunohistochemistry [3]. The treatment consists of chemotherapy protocols, usually with combined medicines, with no evidence that the associated surgical procedure is more effective than chemotherapy alone, except when there is a mass totally or partially obstructing the food transit [10].
However, antineoplastic medicines cause different toxic effects, especially concerning liver parenchyma [16]. Rodaski et al. [17] reported that the hepatotoxicity of chemotherapeutic medicines generally coincides with the increase in serum enzymes such as alanine aminotransferase (ALT) and alkaline phosphatase (AP) [17,18]. Myelotoxicity is another frequent and severe limiting factor of chemotherapy, impairing the treatment effectiveness and increasing the possibilities of metastases [19-21].
Within this context, complementary therapies gain space both as a primary or complementary treatment for cancer patients since they do not present side effects and they stimulate the immune system, improving the quality of life and, consequently, increasing the patient’s survival [22]. iscum album (VA) is the most used plant in the world as a complementary therapy. It is used either in its phytotherapeutic or homeopathic form, presenting selective cytotoxicity effect and being aggressive only against tumor cells and not for normal cells [23], as well as for its immunomodulating and anti-inflammatory action [22]. Valle et al. [24] describe the in vitro selective cytotoxicity of ultra-diluted VA extracts when added to cell cultures of mammary adenocarcinoma and mesenchymal stem cells. This medicine’s cytotoxic activity was at least five times greater in the adenocarcinoma cells than in normal cells, suggesting a higher predilection for tumor cells by the medication.
Kirsch [25] reports a case in which he used VA (Iscador® M) extract as the only modality for the adjunctive treatment of the post-operative treatment of metastatic melanoma. He found the treatment to be extremely effective and very well tolerated in this patient, resulting in the complete remission of the neoplasia. Lefebvre [26] associated VA with the traditional chemotherapy in dogs and observed that the associated therapies decreased the total treatment time, reducing the chemotherapy side effects, such as leukopenia. Valle et al. [27] also described the successful treatment of transmissible venereal tumors using the VA homeopathic therapy. Therefore, the objective of this work was to report the case of a domestic feline, diagnosed with lymphoblastic lymphoma, immunophenotype Type B, who was treated with the ultra-diluted medicines VA and Magnesia phosphorica, and showed remission of the disease.
Materials and Methods
An 11-year-old male Siamese breed feline (Figure 1) weighing 3.8 Kg was attended at the Veterinary Hospital of UNIP, by the service of Natural Medicine, in February 2016. The animal presented a history of weight loss, apathy, lack of appetite, successive emetic episodes, and abdominal pain for three weeks. The animal showed 4% dehydration in physical examination, severe pain on abdominal palpation, normal mucous membranes, the temperature at 38oC, cardiac auscultation compatible with age and species, slightly increased respiratory rate, and lymph nodes of standard size and consistency for age and species. Laboratory tests (complete blood count, urea, creatinine, ALT, and alkaline phosphatase), FIV/FeLV tests, abdominal ultrasound imaging, digestive endoscopy, biopsy, and immunohistochemistry were requested

Figure 1: Feline, male, 11 years old, Siamese breed.
Results
No alteration worthy of note was detected at laboratory tests. Most parameters were within the normal range for age and species in question. Creatinine was the only one altered, confirming the previous diagnosis of chronic kidney disease (Creatinine 2.45mg/dL). FIV/FeLV tests were negative. A mass was visualized in the stomach, in the fundus region (Figure 2a and 2b) at the ultrasound, causing an acoustic shadow. The animal was then referred for a gastric endoscopy, which resulted in the visualization of a mass of approximately 4.6 cm (Figure 3d) and an ulcerated region (Figure 3e) associated with it. The result of the biopsy was from a plasmacytic infiltrate (Figure 4). The immunohistochemistry analysis showed expression of CD79a, whereas no expression of AE1AE3, MUM1, CD3, tryptase, and C-Kit was detected. Therefore, it was concluded that the immunohistochemical and morphological profiles of the piece evaluated favor the diagnosis of immunophenotype B lymphoblastic lymphoma. An injectable homeopathy treatment was chosen, mainly because it is easy to handle, and there are no side effects to the treated patient. Viscum album at different concentrations was used such as D3, D6, D9, D12, which were daily administered in subcutaneous combinations, SID, in the following order: Day 1 – VAD3 + VAD6; Day 2 – VAD9 + VAD12; Day 3 – VAD30 + VAD3, and Magnesia phosphorica D35, one ampoule, subcutaneously, SID. Both medicines were used for 122 consecutive days.
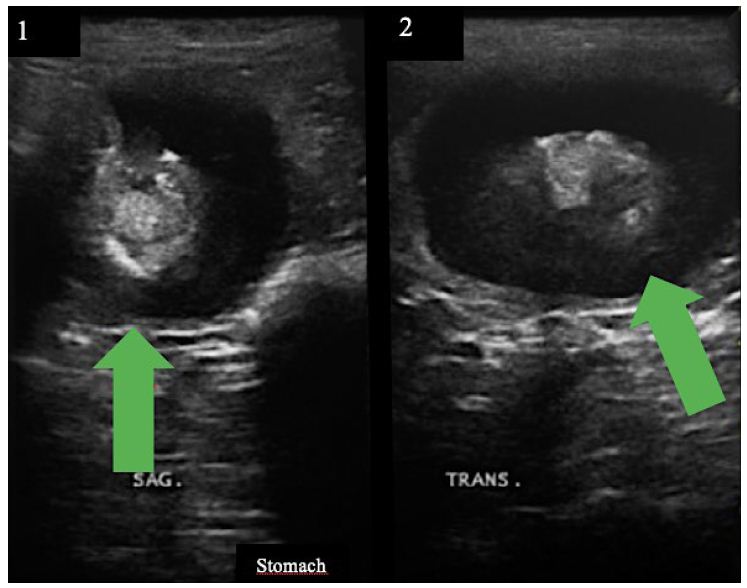
Figure 2: (a) Stomach US (sagittal section) showing a mass of approximately 3.6 cm in diameter, indicated by a green arrow; (b) Stomach US (transversal section) showing a mass of approximately 4.9 cm in diameter.
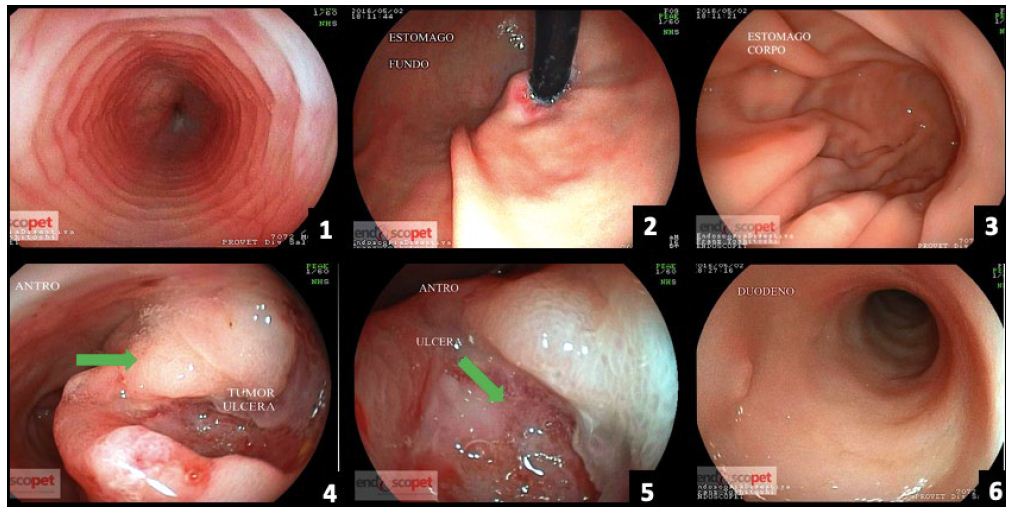
Figure 3: (a) Esophagus; (b) Stomach (fundus); (c) Stomach (body); (d) Stomach tumor (antrum) – ulcerated tumor lesion; (e) Stomach – ulcerated antrum region; (f) Duodenum.
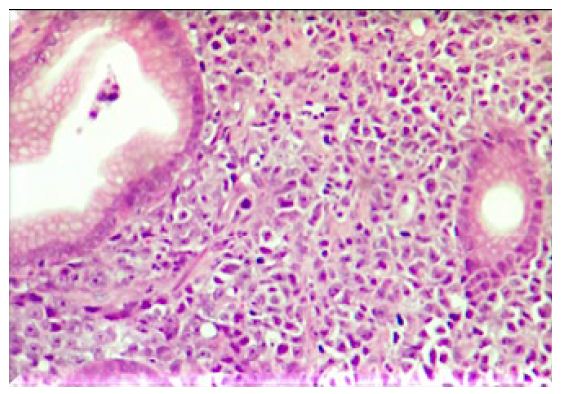
Figure 4: 40X image – Plasma cell infiltrates observed.
The cat’s tutor reported that the animal started eating spontaneously again right in the first week after the beginning of the treatment. The tutor also reported that the pain had reduced after the third application of the proposed medicines. Further evaluation through imaging tests was performed after 122 days. The abdominal US was done again (Figure 5), and the alterations previously visualized were not observed. The following observation was recorded: the stomach wall was within the normal range (0.23 cm), with no ultrasound evidence of abnormalities on this exam. In the images visualized by endoscopy (Figure 6), the stomach had a clear and transparent mucous lake, with preserved shape and architecture. Mucous membranes, rugae of the mucosa, and gastric body showed typical shapes and features to the endoscopic macroscopy. A small sessile polyp (Figure 6d and 6e) was present between the body and antrum canal on the left wall. The antrum canal had a smooth surface and was a little hyperemic. No tissue proliferation was identified in a previous exam. The duodenum showed preserved shape and caliper, and the velvety mucous had a light pink color. A new fragment was collected for histopathological examination from the same previously injured site, which showed intraepithelial granular lymphocytes, non-malignant lymphoplasmacytic infiltrate (Figure 7). The animal was follow-up until the moment of its death due to a kidney disease preexisting to the lymphoma treatment. The death occurred 24 months after the treatment, without signs compatible with the relapse of the lymphoma.
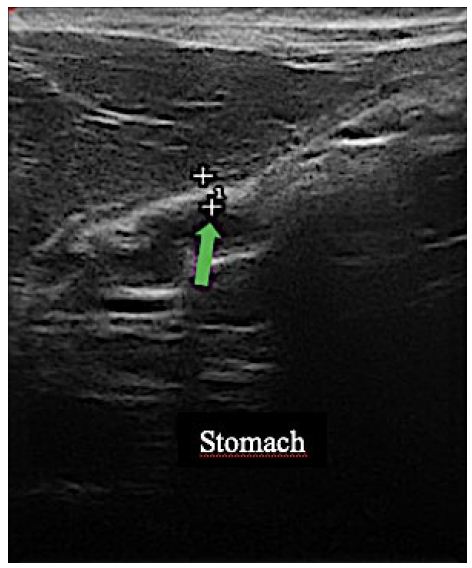
Figure 5: Stomach wall thickness (0.23), indicated by the green arrow, within the normal range for age and species.
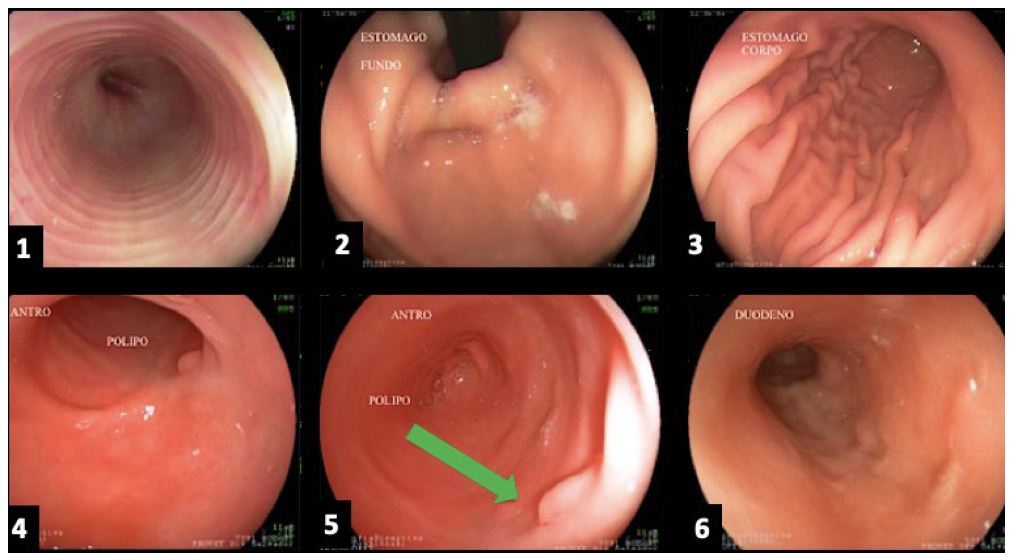
Figure 6: (a) Esophagus; (b) Stomach (fundus); (c) Stomach (body); (d) and (e) Stomach (antrum) with the presence of a polyp, indicated by a green arrow; (f) Duodenum.
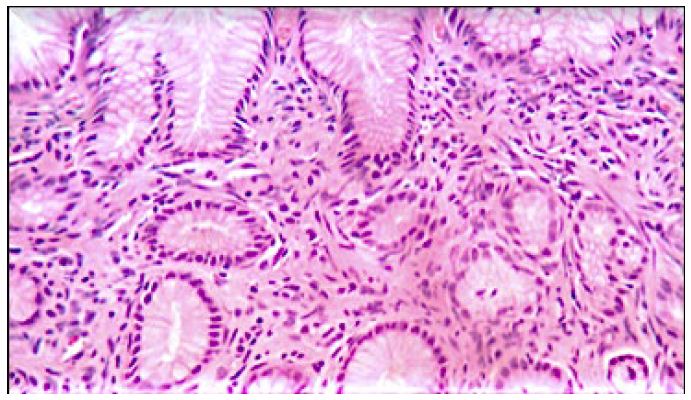
Figure 7: 40X Image – Lymphoplasmocyte infiltrate.
Discussion
Lymphoma is one of the most frequent hematopoietic neoplasias in domestic cats [12]. In cats, alimentary lymphoma is responsible for approximately 50% of all lymphoma cases and is considered as the malignant neoplasm most responsive to chemotherapy [28]. However, several side effects are reported, as previously described.
The alimentary lymphoma is one of the most prominent neoplasms within feline oncology, as it is a relatively aggressive tumor and, in most cases, of difficult treatment [9]. The National Cancer Institute Working Formulation (NCIWF) classified the feline alimentary lymphoma as high, intermediate, and low grades, the latter usually affecting the diffuse form of the disease and was the first type to be described. A less described form is the lymphoma of large granular lymphocytic cells, which is subdivided into immunoblastic and lymphoblastic [29].
The case here reported corroborates with Birchard [14] and presented more severe and acute clinical signs, in addition to a fast onset and progression, being within 80% of cats with this neoplasia. However, in contrast to Birchard [14], the animal evaluated presented the lesion in the fundus region of the stomach and not in the intestinal region. Therefore, no thickening of the intestinal loops or hepatosplenomegaly was recorded in this study. According to Pohlman et al. [30], alimentary lymphoma affects the stomach, small intestine, and large intestine in 24, 74, and 16% of the cases, respectively. The present case is within 24% of the cases that affect the stomach region.
Most cats with lymphoma have a life expectancy of six to nine months when treated with multiple chemotherapeutic agents, associated or not with surgical or radiotherapy treatments. Approximately 20% of the animals survive for more than a year. The prognosis for FeLVpositive cats is worse than the one mentioned above, and the survival is three to four months. FeLV-negative cats survive longer than those FeLV-positive, reaching 9 to 18 months of life, depending on the anatomical shape [31].
In contrast to Amorim [31], this study describes a feline alimentary lymphoma resolution over four months. It also reports the animal’s survival for 24 months, with no occurrence of clinical signs compatible with the initial pathology and with no administration of chemotherapy medication. The patient was treated using injectable, homeopathic medicines to immunomodulate the organism and generate a selective cytotoxic activity [23,24] through the ultra-diluted medicine VA. Injectable and ultra-diluted Magnesia phosphorica was also administered for the treatment of the tumor microenvironment. This protocol confirmed the effectiveness of the unconventional therapy in treating gastric lymphoma and showed excellent results, such as being minimally invasive, no side effects, and low cost compared to the therapies of choice for the treatment of this disease.
The main prognostic factor is the initial response to chemotherapy and if remission occurs. Cats with a good initial response to the chemotherapeutic treatment and with total remission usually survive, on average, one year. However, this case report describes the treatment using injectable homeopathy in which the patient demonstrated excellent response, with remission of the tumoral mass, with no side effects, and with the reestablishment of its health in 122 days. Also, the animal had 28 months of survival until the moment of this report.
Conclusions
In conclusion, the present case report offers one more option for successfully treating feline gastric lymphomas so as not to produce side effects to the patient, be minimally invasive, and of low cost when compared to conventional treatments. However, more studies are still necessary to better elucidate the mechanism of action of this medication class.
References
- COURT EA, WATSON ADJ, PEASTON AE (1997) Retrospective study of 60 cases of feline lymphosarcoma. Australian Veterinary Journal 75: 424-427. [crossref]
- GABOR LJ, MALIK R, CANFIELD PJ (1998) Clinical and anatomical features of lymphossarcoma in 118 cats. Australian Veterinary Journal 76: 725-732. [crossref]
- NORSWORTHY GD, GRACE SF, CRYSTAL MA, TILLEY LP (2011) The feline patient. 4. ed. Iowa: Wiley – Blackwell. 1073 p.
- STÜTZER, B Karin Simon, Hans Lutz, Monir Majzoub, Walter Hermanns, et al. (2011) Incidence of persistent viraemia and latent feline leukaemia virus infection in cats with lymphoma. J Feline Med Surg 13: 81-87. [crossref]
- WOLDEMESKEL M (2020) Primary Cardiac Lymphoma in a Cat. J Comp Pathol 174: 34-38.
- HARTMANN K (2012) Feline leukemie virus infection. In C. E. Greene (Ed.), Infections dieseases of the dog and cat. Missouri, USA: Elsevier.
- LUTZ H, ADDIE D, BELÁK S, BOUCRAUT-BARALON C, EGBERINK H, et al. (2009) Feline leukaemia. ABCD guidelines on prevention and management. Journal of Feline Medicine & Surgery 11: 565-574. [crossref]
- COTTER SM, HARDY JUNIORR WD, ESSEX M (1975) Association of feline leukemia virus with lymphosarcoma and other disorders in the cat. Journal of the American Veterinary Medical Association 166: 449-454. [crossref]
- BADO AS (2011) Alimentary lymphoma in cats. Undergraduate thesis (Undergraduation in Veterinary Medicine) – Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil. 38p.
- WILSON HM (2008) Feline Alimentary Lymphoma: Demystifying the Enigma. Topics in companion animal medicine 23: 177-184. [crossref]
- COUTO CG (2000) Advances in the treatment of the cat with lymphoma in practice. Journal of Feline Medicine and Surgery 2: 95-100. [crossref]
- ORTIZ BC, SOARES CA, GOMES VR, SECCHI P, SCHULZ JÚNIOR FJ, et al. (2019) Lymphocytic alimentary lymphoma in a feline: therapy with lomustine and prednisone – Case report. Pubvet 13: 1-5.
- MORRIS J, DOBSON J (2001) Small animal oncology. Londres: Blackwell science. 315.
- BIRCHARD SJ (2008) Saunders Manual: small animal practice. 3. ed. Sao Paulo: Roca. 2048.
- PATTERSON-KANE JC, KUGLER BP, FRANCIS K (2004) The possible prognostic significance of immunophenotype in feline alimentary lymphoma: a pilot study. J Comp Pathol 130: 220-222. [crossref]
- BERGER C, HUG M, GYSIN C MOLINARI L, FREI M, BOSSART W, et al. (2007) Distribution patterns of beta- and gamma-herpesviruses within Waldeyer’s ring organs. Med. Virol 79: 1147-1152. [crossref]
- RODASKI S, WERNER J (2008) Skin neoplasia. In: DALECK, C.R., DE NARDI, A.B., RODASKI S (Eds) Oncology in dogs and cats. Sao Paulo: Roca. 253-279.
- ARAUJO GG (2009) Feline lymphoma. 45 p. Undergraduate thesis (Undergraduation in Veterinary Medicine) – Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil.
- HERNANDEZ L, PINYOL M, HERNANDEZ S, BEA S, PULFORD K, et al. (1999) TRK-fused gene (TFG) is a new partner of ALK in anaplastic large cell 9 lymphoma producing two structurally different TFG-ALK translocations. Blood 94: 3265-3268.
- NELSON RW, COUTO CG Small Animal Internal Medicine. 4th ed. Rio de Janeiro: Elsevier. 1468.
- LANORE D, DELPRAT C (2004) Anticancer chemotherapy. São Paulo: Roca. 53-78.
- Rostock M. (2020) Die Misteltherapie in der Behandlung von Patienten mit einer Krebserkrankung [Mistletoe in the treatment of cancer patients]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 63: 535-540.
- CARVALHO AC (2015) Antineoplastic activity of Viscum album (L) in experimental tumors: a critical review and experimental study in Ehrlich tumor. Doctorate Dissertation in Environmental and Experimental Universidade Paulista.
- VALLE ACV, LIMA L, BONAMIN L, BRUNEL H, BARROS A, et al. (2020) Use of Viscum album in the Integrative Treatment of Cholangiocarcinoma in a Dog (Canis familiaris) – Case Report. Adv Complement Alt Med 5: 476-481.
- KIRSCH A (2007) Successful treatment of metastatic malignant melanoma with Viscum album extract (Iscador M). J Altern Complement Med 13: 443-445. [crossref]
- LEFEBVRE GNF, BONAMIN LV, OLIVEIRA CM (2007) Treatment of canine transmissible venereal tumor (TVT) using Viscum album in combination with chemotherapy. Revista Clinica Veterinária 12: 78-86.
- VALLE ACV, SIBATA MN, ANDRADE RV, CARVALHO AC (2019) Homeopathy for the Treatment of Transmissible Venereal Tumor (TVT) in a Mixed-Breed Female Dog. Adv Complement Alt Med 5: 422-424.
- LIPP VB (2008) Lymphsarcome in dogs Undergraduate thesis. Faculty of Veterinary Medicine, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil.
- LINGARD AE, BRISCOE K, BEATTY JA, MOORE AS, CROWLEY AM, et al. (2009) Low-grade alimentary lymphoma: clinicopathological findings and response to treatment in 17 cases. Journal of Feline Medicine and Surgery 11: 692-700. [crossref]
- POHLMAN L, HIGGINBOTHAM ML, WELLES E, JOHNSON C (2009) Immunophenotypic and Histologic Classification of 50 Cases of Feline Gastrointestinal Lymphoma. Veterinary pathology 46: 259-268. [crossref]
- AMORIM FV, ANDRADE VV, SOUZA HJM, FERREIRA AMR (2006) Meadistinal lymphona in cats – case report. Clínica Veterinária São Paulo 63: 68-74.