A technique for revealing surface morphology of human cervical cancer cells has been developed to facilitate early diagnostics of a pre-cancer and cancer cells under reflected light microscopy. The offered method was borrowed from optical microscopy of a solid state surface where the Metallographic Inverted Microscopy (MIM) are usually used. Unlike common accepted transmitted light microscopy for biological applications MIM technique allows to reveal a morphology and topology of a biological cells surface without any treatment by chemicals (fixing, staining, drying, freezing et al). The MIM method was demonstrated by analyzing fresh native smears from epithelium of uterine neck. MIM micrographs of 167 patients with diagnosis cervical cancer allow visualizing on the cancer cells surface numerous of the Light Reflective Formations (LRF). It is supposed that LRF are connected with exocytosis on the cell membrane. For smears of cervix epithelium throughout the field of view of a microscope numerous ballooning-outs , which have a mean size from 0.1-0.5 to 1.2 -1.3 mkm, are seen located on the cell surface. It is accepted that in result of a cancer cell metabolism a granules or vesicles originate inside of cell and move towards cell surface to release its contents. Visualization of such morphological formations has however been limited, partly due to the difficulties with imaging native living or structurally intact cells because convenient transmitted light microscopy technique do not reveal surface cell features which are usually removed after fixing, drying and other treatments of smears. We suppose that offered method to visualize cell topography in air without fixation and dehydration may be alternative and complementary to Pap-test.
Keywords
Cervical Cancer, Reflected Light Microscopy, Cell Membrane, Smears.
Introduction
Cervical cancer is the second most common cancer in women worldwide. More than 80% of cervical cancers occur in the developing world where the least resources exist for management. Most cases of cervical cancer can be prevented by screening measures which detects precancerous lesions and subsequent treatment. Indeed in many countries where screening programs have been established, the morbidity and mortality of cervical cancer have been sharply reduced. Unfortunately many developing countries have not necessary resources and infrastructure for screening program so the annual cases of cervical cancer accounts 493 000 and deaths about 273,500 [1].
Currently, optical microscopy techniques are the primary method for cell visualization, with microscopic characteristics of cells traditionally used for diagnosis and classification of cancers [1]. However, because the differences in characteristics can be subtle, accurate detection can be challenging and ambiguous [2,3]. Particularly at present time the key accepted technique in the world to detect cervical cancer cells is Papanicolau (Pap) test [1]. To realize Pap-test need to visualize pathological peculiarities of nuclear, cytoplasm and cell shape. For this purpose need to fix smears, and next many step treatments by chemicals (staining by dyes, washing, dehydration etc). This procedures lead to destruction of alive cells, removing very important diagnostic signs and artifacts emergence. Besides there is another problem with analyzing Pap smears, connected with drying changes in cells due to delays in applying the fixing procedure. It is very difficult to evaluate smears that have dried in air and still distinguish abnormal cells. Sample handling and its evaluation of Pap-test is a time consuming procedure and demands a sophisticated testing infrastructure and highly trained professionals to evaluate the cytological test as a result, the vast majority of women in the developing world do not have access to life-saving screening programs [4]. To reach the main goal of cervical cancer screening we need to find an accessible, simple, low cost, highly sensitive and fast complementary or substitutive method to detect cervical cancer cells instead of the Pap-test.
One of lacks of modern clinical cytology is use basically transmitted light optical microscopes. But clinical cytology almost does not pay attention to such important element of a cellular structure as features of membrane topography. The last can be result of infringement of a metabolism of all whole cell or only components of the membrane [4]. Structural changes of an external membrane can arise due to external physical and chemical factors, or thank to internal response to immune stresses. Meanwhile in the standard clinical microscopy techniques the surface of cells in smears is not investigated. According to the accepted rules smears of a patient undergo many step chemical processing. In so way rather valuable probably diagnostic signs of pathological cells will removed. Quite probably that as a result of processing occurrence in structure of objects of the various artifacts distorting the analysis [5].
For partial elimination of above mentioned shortcomings it is offered to use the optical microscopes working in reflected light. Besides it is possible to notice that received pictures of a cellular structure in an offered method of the analysis sometimes with such features which can be received only using a raster electronic microscope. At slanting illumination of a surface of analyzed cells, fine pattern of their structure get volume perception that facilitates decoding of results of the analysis.
Another high resolution tools such as the atomic force (AFM) or scanning electron microscopes (SEM) let us to see cell membrane and organelle more in detail but they operate in environments too harsh for living cells that leads to its damage. Typically, cells have to be treated with chemicals for fixation, dehydration and drying, and in the case of the SEM, coated with metal. Recently proposed Bioimprint method [6] is a soft lithography replication technique used to create a time-shot replica of biological cells in a polymer during curing. Cells at near-native states can be imprinted into a photo-curable polymer, and the imprint analyzed without imaging directly impacting on cell viability. A technique for permanently capturing a replica impression of biological cells has been developed to facilitate analysis using nanometer resolution imaging tools, namely AFM. The transfer of nanometer scale biological information is presented as an alternative imaging technique at a resolution beyond that of optical microscopy.
James K. Gimzewski, Jianyu Rao (Nat. Nanotechnol. DOI: 10.1038/nnano.2007.388) find that AFM can be used to distinguish metastatic cancer cells from normal cells on the basis of stiffness rather than shape. The cancer cells are significantly squishier than the normal cells, and the method promises to improve cancer diagnostics. The researchers find that metastatic cancer cells are nearly four times softer than normal cells.
This paper presents an alternative method for studying biological cells using a optical metallographic inverted microscope (MIM) technique, to enable imaging of the surface topography of human cervical cancer cells. Here we present a new optical-probe technique based on light-scattering microscopy that is able to detect precancerous and early cancerous changes in cell-rich epithelia.
Methods
Smears from uterine neck were collected for patients with diagnosis cervical cancer. Smears were collected by Ayre’s spatula after exposing the cervix by a Cusco’s speculum. Samples collected were transferred to glass slides. Glass slides were preliminarily cleaned thoroughly in a 2 vol.% detergent followed by repeated washing in pure deionized water. Two set of slides were prepared for each patient and fixed by 95% ethanol. Relevant information was obtained from the patient and recorded on a specially designed proforma. The first sets of marked slides were then sent to cytology laboratory to view at high magnification with MIM without fixing and any treatment by dyes. This native smears has been observed under optical reflected microscope “Neophot-2”. For each smear under investigation from 4 to 10-13 field of view was captured. In this case we watched a morphology and topology of cell membrane and captured these images on digital photocamera. This measurement has been performed in Institute of Electronics Uzbek Academy of Science. Both practitioner and patient can then see smears image in color. The results are then used as a basis for prescribing supplements.
The second set of smears was prepared on glass and fixed by 95% ethanol and stained with Papanicolaou dyes and were then sent to Pathology Institute and each slide was then carefully examined by a cytopathologist to distinguish normal cells from leased one. Relevant information was recorded on a specially designed proforma on PC and was marked on the slides. It was clearly specified whether smear was satisfactory or not. Slides showing some abnormal changes in the cellular pattern were further scrutinized by a cytopathologist. Images has been observed on optical microscope “JENAMED-2” and captured by digital high Resolution Microscopy Camera AxioCam MRc Rev. 3 FireWire.
Results and Discussion
Cervical normal and cancer cells was evaluated both traditional Pap-test and new MIM technique. On the figure 1 we can see image of normal cell (control smears) surface captured by MIM technique. with pronounced nuclear. Three kinds of multilayer epithelial cells were observed in control smears (Figure 1 b). Epithelial cells of upper layer had polygonal shape with smoothed cell surface and size about 30-60 mkm with small (about 6 mkm ) nuclear. Cells of more deep layers had smaller size. Parabasal cells of cervix epithelial layer had round shape with size 12-17 mkm and coarse nuclear enclosed by cytoplasm. Minor auto flora around cells was observed. Fine granularity was observed in cell cytoplasm of upper and deeper epithelial layers.
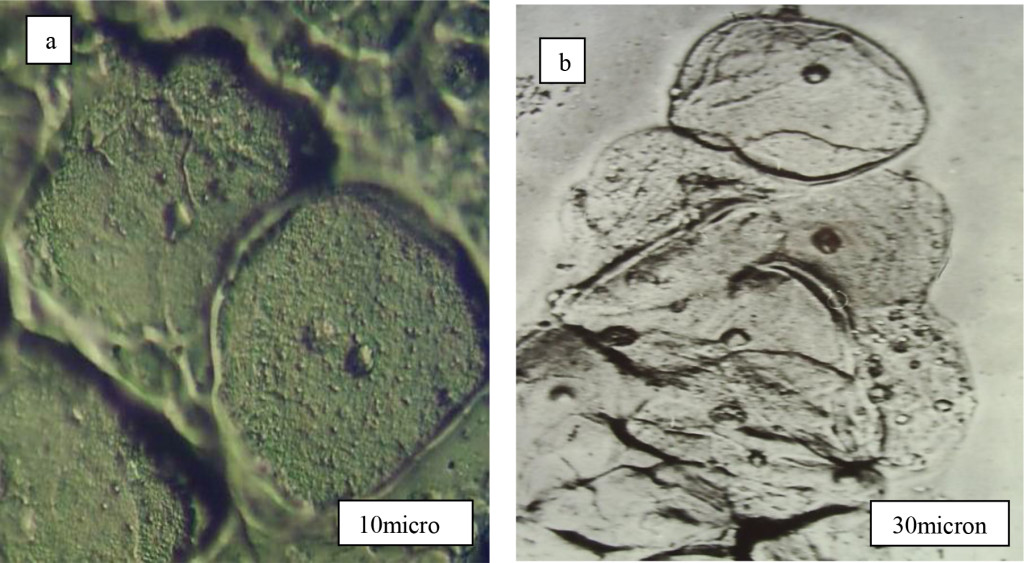
Figure 1. Control cervical smears with normal epithelial cells. a-separate cells, b-parabasal cells of many layer pavement cervix epithelium.
In the case of traditional Pap-test cervical smears was fixed and stained by dyes (Figure 2). Here we can basically see content of cells (nuclear, nucleolus, cytoplasm, vacuole etc) and their shape. Hyperchromic pronounced enlarged nuclear rounded shape, anomalous ratio between nuclear and cytoplasm usually is main diagnostic peculiarity of Pap-test to distinguish cancer and normal cells. But Pap-test do not permit to see cancer cell membrane destruction due to removing any diagnostic signs on cell membrane. Offered here MIM technique deals with native smears and let us to see just membrane morphology in reflected light. Indeed for cervical cancer cells we observed ballooning–out of cell membrane with specific distribution around nuclear (Figure 2). This ballooning-out formations have high light reflectivity due to its content and smooth shape and named by us Light Reflecting Formations (LRF) (Figure 2.). They have rather small diameter (0.3-0.5 microns) and consequently were accurately observed only at big optical magnifications (800-1000×).
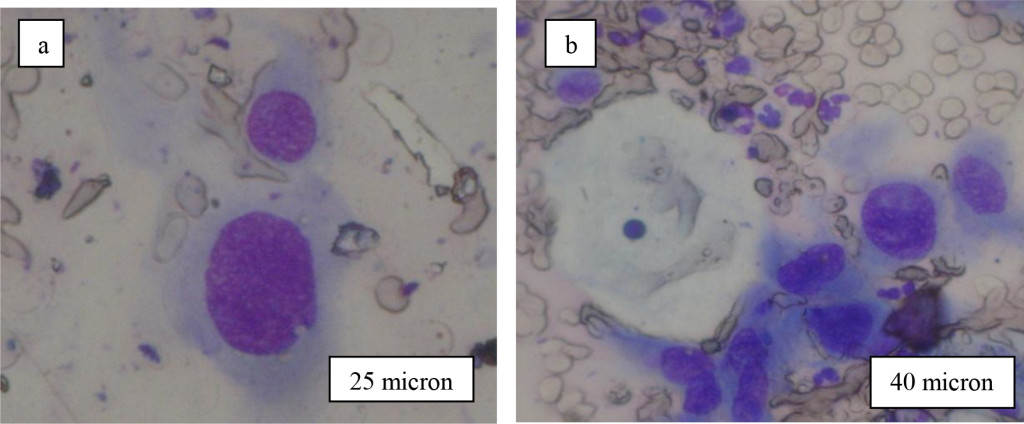
Figure 2. Typical view of cervical cells after staining by dyes (Pap-smears).
For cervical epithelial cells two rinds of LRF arrangement on cell membrane are possible. The first variant is connected with presence of LRF only on polygonal cells from the top layer of multicellular epithelium (Figure 1). Thus LRF can settle down on a surface of cell membranes as by the piece (them it is possible even to count (Figure 3 a,b), and in the form of high density LRF associations (Figure 4а, b).

Figure 3. Cervical cells of upper epidermal layer of patients with diagnosis Cr. coli uteri
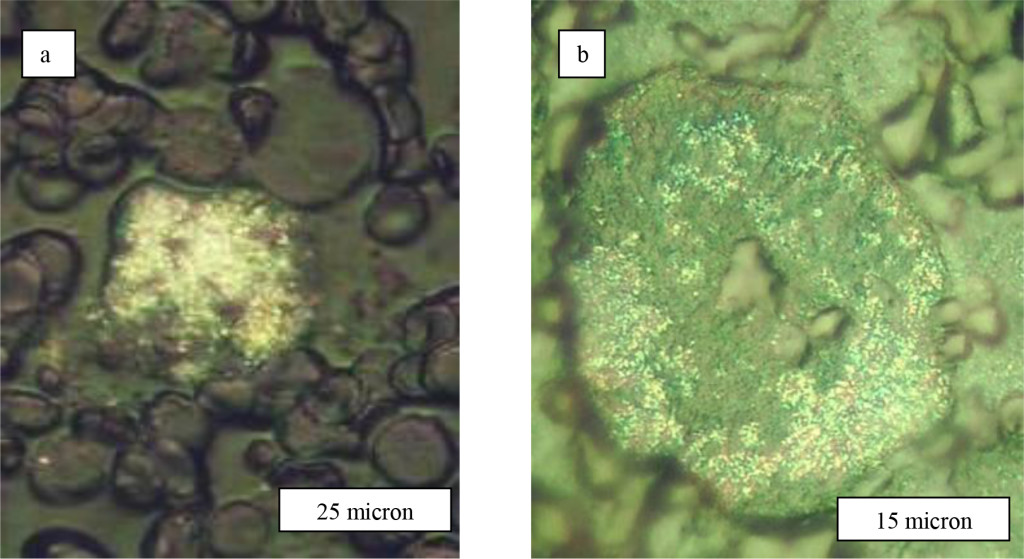
Figure 4. Assosiations of LRF on cervical cells of upper epidermal layer of patients with diagnosis а-Cr. coli uteri and b-Cr. corporis uteri T1N0M0
The second really observable variant of LRF in cervical smears is connected with cells of intermediate and parabasal layers (Figure 5а). In intermediate roundish cells besides LRF it is sometimes shown clumpy granularity of cytoplasm and excentricly located nuclear (Figure 5а). In parabasal cells of the bottom epidermal layers from cervix LRF in size 12-15 microns basically settle down separately (Figure 5b). Thus, experimental data indicate that for cervical cancer cells LRF are observed on polygonal top layer epidermal cells or only on bottom layer cells. In the first case for some patients the surface of epithelial cells contained isolated LRF which was possible even to count them by the piece (Figure 3). But for other women similar cells have been entirely was choked up LRF (Figure 5а). It is not clear yet what is reason so difference in LRF arrangement on a cervical cancer cell surface, and density its distribution along membrane. The nature of LRF occurrence and a its chemical compound is not clear yet too The analysis of the literature let us to assume that observed structural phenomenon in cervical smears may be connected with strengthened vacuolisation of cytoplasms and intensive aerobic glycolysis (fermentation) in cancer cells [7].
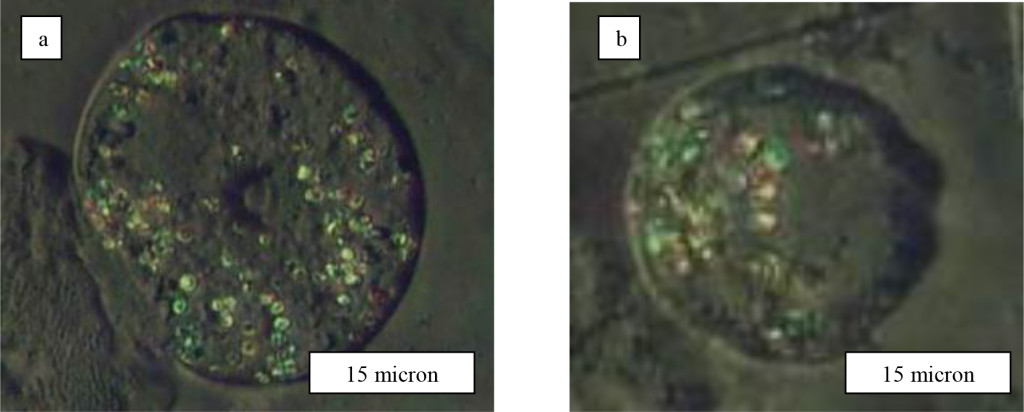
Figure 5. Neoplastic intermediate (а) and parabasal (b) cells of many layer epithelium of cervix.
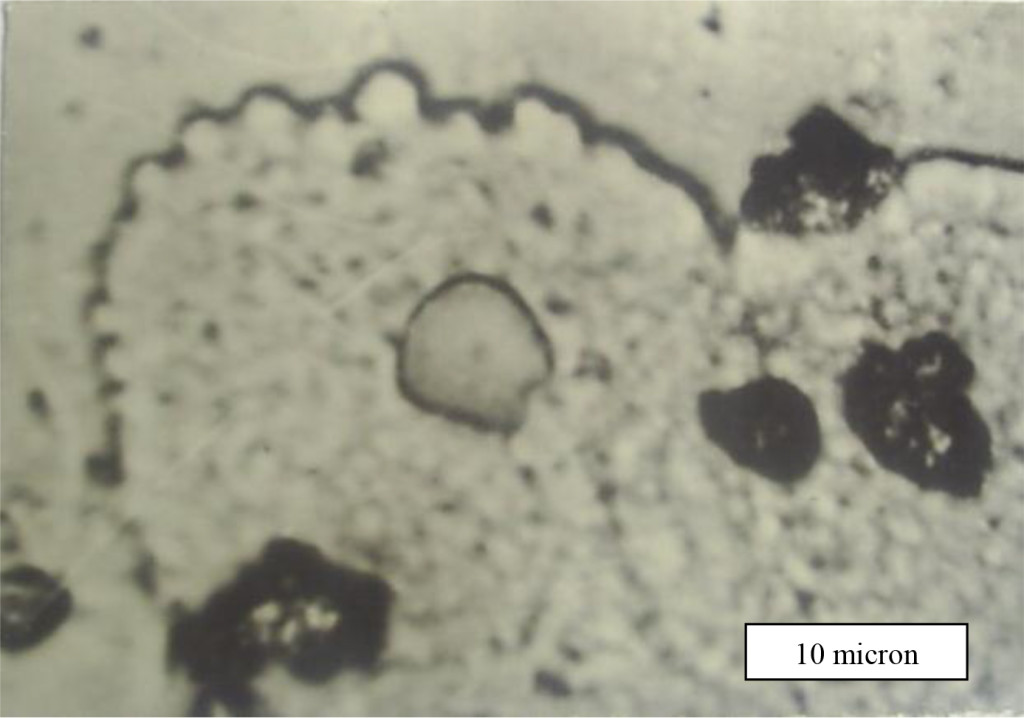
Figure 6. Ballooning–out of cervical cancer cells membrane.
Really, in the course of experiment we accurately observed intensive process of a cell membrane reorganization of cervical cancer cells. The location of the LRF on the cell membrane is shown in Fig. 4 too for patients with late stage of disease, where they are seen predominantly concentrated at areas around the nucleus. The diameter of non-dysplastic cell nuclei is typically 5 mkm, whereas dysplastic nuclei can be as large as 10 mkm and more.
The nature and mechanism formation these features of cancer cells are not understood up to now but they are potentially associated with exocytosis. It is accepted that in result of a cancer cell metabolism a granules or vesicles originate inside of cell and move towards cell surface to release its contents. Visualization of such morphological formations has however been limited, partly due to the difficulties with imaging native living or structurally intact cells because convenient transmitted light microscopy techniques do not reveal surface cell features which are usually removed after fixing, drying and other treatments of smears.
Much is yet to be known about the nature of cervical cancer cells and until now there has not been a reliable, simple method for cervical cell testing. We suppose that offered MIM method to visualize cell topography in air without fixation and dehydration may be alternative and complementary to Pap-test. Being able to directly view membrane structures regulated by exocytosis will enable researchers to analyze the secretory nature and response of cells, yielding insights into drug responses and effects [6]. Considerable variability in the sizes of LRF, as well as dynamic formation and grouping of these structures around the nucleus, illustrates that cells have diverse morphologies.
Conclusion
A technique for revealing surface morphology of human cervical cancer cells has been developed to facilitate early diagnostics of a pre-cancer and cancer cells under reflected light microscopy. The offered method was borrowed from optical microscopy of a solid state surface where the Metallographic Inverted Microscopy (MIM) is usually used. Unlike common accepted transmitted light microscopy for biological applications MIM technique allows to reveal a morphology and topology of a biological cells surface without any treatment by chemicals (fixing, staining, drying, freezing et al). The MIM method was demonstrated by analyzing fresh native smears from epithelium of uterine neck. MIM micrographs of 167 patients with diagnosis cervical cancer allow visualizing on the cancer cells surface numerous of the Light Reflective Formations (LRF). It is supposed that LRF are connected with exocytosis on the cell membrane. For smears of cervix epithelium throughout the field of view of a microscope numerous ballooning-outs, which have a mean size from 0.1-0.5 to 1.2 -1.3 mkm, are seen located on the cell surface. It is accepted that in result of a cancer cell metabolism a granules or vesicles originate inside of cell and move towards cell surface to release its contents. Visualization of such morphological formations has however been limited, partly due to the difficulties with imaging native living or structurally intact cells because convenient transmitted light microscopy techniques do not reveal surface cell features which are usually removed after fixing, drying and other treatments of smears. We suppose that offered method to visualize cell topography in air without fixation and dehydration may be alternative and complementary to Pap-test.
Acknowledgement
This work has been supported by Grant № X11-001 of Coordination Center for Science and Technology Republic of Uzbekistan.
References
- Koss LG (1989) The Papanicolaou test for cervical cancer detection. A triumph and a tragedy. JAMA 261: 737–743. [crossref]
- Hamby L (2002) Gene expression patterns and breast cancer. Cancer Genetics News, Spring 4: 1
- Palaoro LA, Blanco AM, Gamboni M, Rocher AE, Rotenberg RG (2007) Usefulness of ploidy, AgNOR and immunocytochemistry for differentiating benign and malignant cells in serous effusions. Cytopathology 18: 33–39.
- Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, Gordillo-Tobar A, Levin C, et al. (2005) Cost-effectiveness of cervical-cancer screening in five developing countries. N Engl J Med 353: 2158-2168. [crossref]
- DaCosta RS, Wilson BC, Marcon NE (2007) Fluorescence and spectral imaging. ScientificWorldJournal 7: 2046–2071. [crossref]
- Muys JJ, Alkaisi MM, Melville DOS, Nagase J, Sykes P, et al. (2006) Cellular transfer and AFM imaging of cancer cells using Bioimprint. Journal of Nanobiotechnology 4: 1–10.
- Mirabal YN, Chang SK, Atkinson EN, Malpica A, Follen M, et al. (2002) Reflectance spectroscopy for in vivo detection of cervical precancer. J Biomed Opt 7: 587–594. [crossref]