DOI: 10.31038/CST.2018311
Abstract
Background: Thanks to the increased effectiveness of the three pillars of cancer therapy i.e. early diagnosis, targeted surgery and chemotherapy, physician are now aiming at a now goal: improve quality of live of patients during and after cancer therapy
Objectives: This review article aims to identify clinical evidence of the effectiveness that MI and IP6 might have on QoL in cancer patients.
Methods: literature search was performed on MEDLINE, EMBASE, PubMed, Research Gate and Google scholar for studies published in English up to November 2017. We used the following combination of medical subject headings, terms and free text words: ‘inositol’, ‘quality of life’, ‘cancer’.
Conclusions: In conclusion, literature data seams to demonstrate that IP6 and MI are effective in improving QoL of patients undergoing chemotherapy due to breast cancer.
Keywords
Breast cancer, Quality of live, inositol, IP6, phytic acid
Introduction
Nowadays taking advantage of the three main tools that physicians are using to fight cancer, i.e. early diagnosis, targeted surgery and medical treatments (chemotherapy, hormonotherapy, immunotherapy) survival rate has reached a remarkable goal of roughly 65% (ranging from 25% for lung cancer to 87% for prostate cancer) [1, 2].
Such success rate has, with time, forced physicians to face a new challenge: how to improve patients’ quality of life (QoL) without reducing survival rate. Indeed, it has been demonstrated that QoL is an independent predictor with respect to life expectancy [3].
What is for sure is that we cannot simply ask for a reduction in chemo-radiotherapy so to improve QoL, indeed Bonadonna and coworkers demonstrated that as soon as patients receive less than 85% of the planned dose intensity the survival rate significantly decrease [3].
It is worth noting that the relative dose reduction might refer to both an actual reduction in the dosage of the drug used or to a delay in the therapy [4].
Having this in mind, several research groups worldwide are committed in improving patients QoL and therefore, eventually increase cancer treatment effectiveness too.
In this scenario, a major role has been played for several years by inositol(s), mainly myo-inositol (MI) and inositol hexakisphosphate (IP6) [4-6].
In this systematic review, we aim to identify clinical evidence of the effectiveness that MI and IP6 might have on QoL in cancer patients.
M&M Search strategy and data sources
We performed a literature search of MEDLINE, EMBASE, PubMed, Research Gate and Google scholar for studies published in English up to November 2017. We used the following combination of medical subject headings, terms and free text words: ‘inositol’, ‘quality of life’, ‘cancer’. Only clinical trials evaluating the effects of IP6 or IP6+MI as study group in women undergoing radio/chemotherapy for breast cancer were considered eligible.
In addition, reference lists of additional manuscript published were reviewed in order to identify additional eligible studies.
We followed the PRISMA checklist for meta-analysis [7].
Inclusion and exclusion criteria
Articles were critically reviewed for their eligibility in the meta-analysis. Among all the collected articles, clinical trials were identified by reading titles, abstracts and study design to select relevant studies according to inclusion/exclusion criteria.
Inclusion criteria restricted the search to: (a) the population of interest was made of women undergoing radio/chemotherapy due to breast cancer, (b) the intervention was IP6 with or without MI, (c) clear quantitative assessment of both quality of life (QoL) and blood counts. Exclusion criteria were: (a) duplicate publications, and duplicates on different database, (c) review papers and (d) animal studies.
Outcomes of interest
Primary outcomes: Quality of life, Functional status and Symptomatic scale based on the EORTIC questioner. Secondary outcomes: white blood cell and platelet counts.
Data extraction and quality evaluation
The following data were extracted from the selected studies and independently cross-checked by two investigators: general characteristics of the study (first author’s name, country where the study was conducted, study design, number of cases and controls, inclusion/exclusion criteria, type and duration of treatment) and results (means and S.D. for each outcome after intervention from treatment vs control). The quality of reports was evaluated according to the methods recommended by the Cochrane Handbook 5.0.2 [8]. including assessments of the randomization process, allocation concealment, blinding, selection criteria, baseline characters and withdrawal/dropouts.
Statistical analysis
The effect size was measured as the mean difference (MD) between the two treatment groups. A MD less than 0 was considered as a positive size effect for symptomatic scales; MD greater than 0 was considered as a positive size effect for Quality of life, Functional status, with blood cell counts and platelet counts. The heterogeneity analysis of intervention was performed by the Cochran’s Q test and the I2 statistic, using a P value = 0.10. In order to account for heterogeneity across studies, the Der Simonian and Laird random effect model was used to obtain the pooled estimates and their 95% confidence intervals (CIs). Forrest plots were used to visually show the results of the analyses performed.
Meta-analysis was performed by means of OpenMeta [Analyst] software developed by The School of Public Health at Brown University USA. Results were considered statistically significant when the two-sided P value was <0.05.
Results
The flow diagram of the meta-analysis is presented in Figure 1 [7]. Based on the search 11 records were identified. After the screening 6 articles were assessed for eligibility. Following the screening 2 out of 4 papers were included in the analysis.
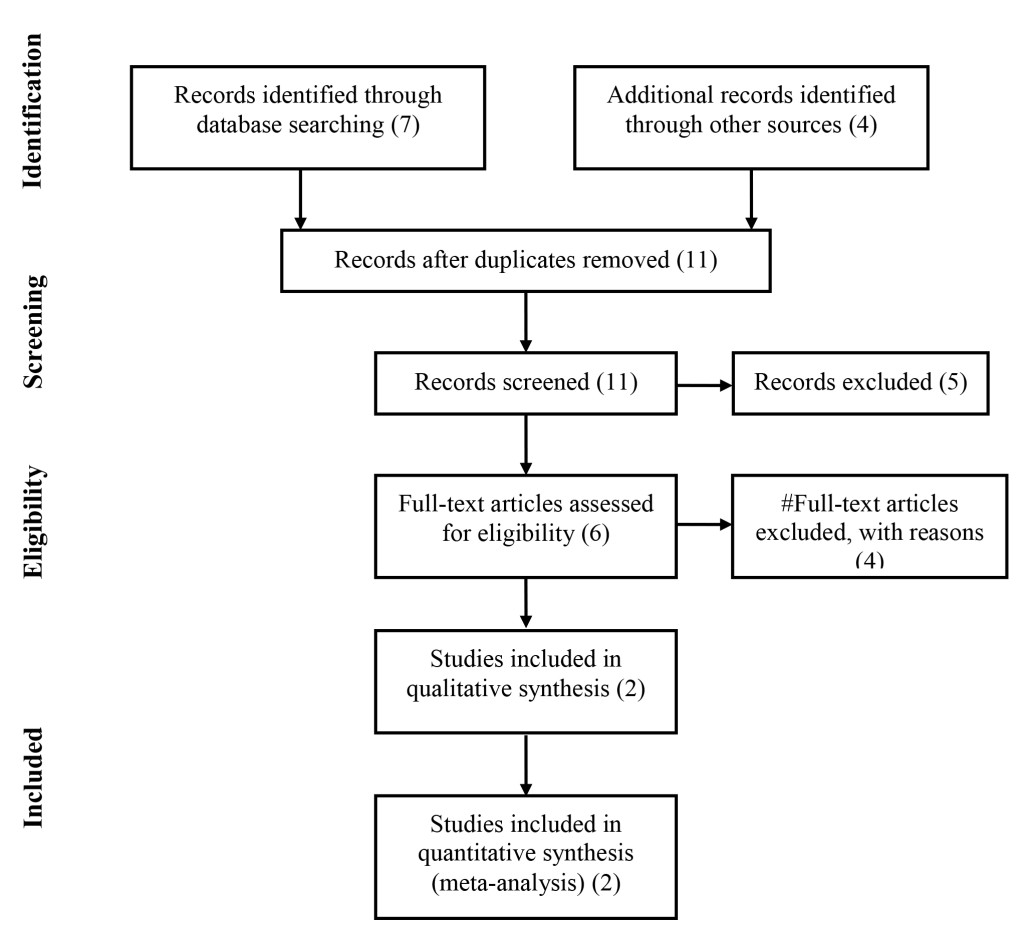
Figure 1.
A brief description of the manuscript matching the inclusion criteria is reported in Table 1.
Table 1.
|
Reference |
Tumor Status |
Chemotherapy |
groups |
outcomes |
| Bacić I et al., 2010 | Ductal invasive BC | 5 fluorouracilepirubicincyclophosphamide | Experimental group IP6+MIControl group Vit C | QLQ30 e QLQ-BR23[28, 29] |
| Proietti et al., 2017 | Ductal BC Stage II-III | cyclophosphamide methotrexato5 fluorouracil | Experimental group IP6 in gelControl group hyaluronic acid in gel | QLQ30 e QLQ-BR23[28, 29] |
Studies were conducted in Croatia [9] or Italy [10] and were published between 2010 and 2017.Treatments administered were IP6 +MI per OS at the dosage of 1.4g twice a day[9] or 5g of a 4% IP6 gel twice a day [10]. Noteworthy both treatments result in the same pharmacokinetic profile [11, 12].The duration of the treatment was 6 months for both studies.
The overall methodological study quality is summarized in Table 2.
Table 2.
| Study | Randomization | Allocation concealment | Blinding | Selection criteria described | Comparable baseline | Withdrawal dropout described |
| BACIC | M | Unclear | N | Y | Y | Y |
| PROIETTI | M | Unclear | Y Double blind | Y | Y | M |
Evaluation according to the methods recommended by the Cochrane Handbook 5.0.2.
M, the method was mentioned, but there was not detailed description;
N, the method was not used in the study;
Unclear, no relevant information was found in the study;
Y, the method was reported with detailed description.
The meta-analysis
In the two selected studies, a total of 17 women received IP6 alone or in combination with MI, and 17 women received control treatments (i.e. Vit-C, Hyaluronic acid gel).
The overall MD estimated from two studies showed a significant improvement for the patients treated with IP6 (+MI) after chemotherapy of the QoL (MD=36.167; 95%CI: 22.047 to 50.288 P= < 0.001) (Figure 2).
Additional improvements were highlighted for the Functional status (MD=33.261; 95%CI: 22.727 to 43.795 P= < 0.001) (Figure 3) and Symptoms Scale (MD= -28.577; -95%CI: 41.476 to -15.678; P= < 0.001) (Figure 4).
In addition to the data obtained from the EORTIC QLQ-C30 and QLQ-BR23 the blood counts results showed that IP6, eventually in association with MI, was able to reduce WBC drop after chemotherapy (MD= 3.579; -95%CI: 2.083 to 5.074 P= < 0.001) (Figure 5).
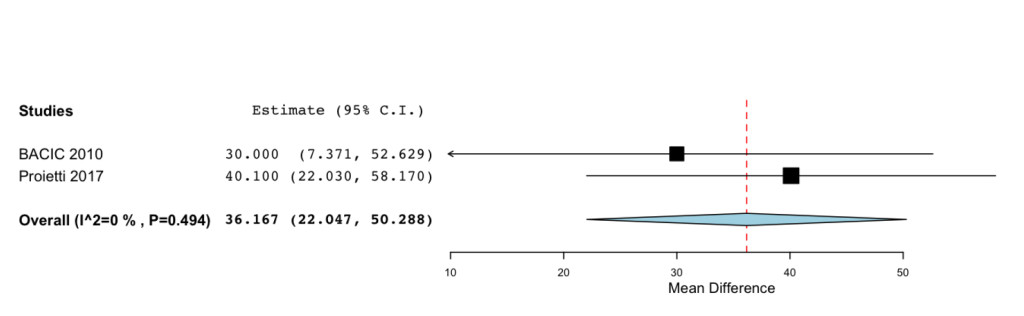
Figure 2. Forest plot showing effect sizes (mean difference (MD), 95% confidence interval (CI)) for QoL in women undergoing chemotheraphy for breast cancer treated with IP6 (+MI).
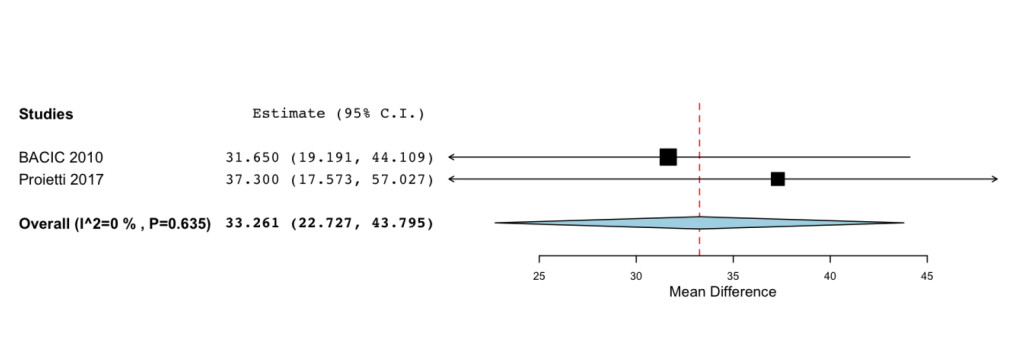
Figure 3. Forrest plot showing effect sizes (mean difference (MD), 95% confidence interval (CI)) for Functional Status in women undergoing chemotheraphy for breast cancer treated with IP6 (+MI).
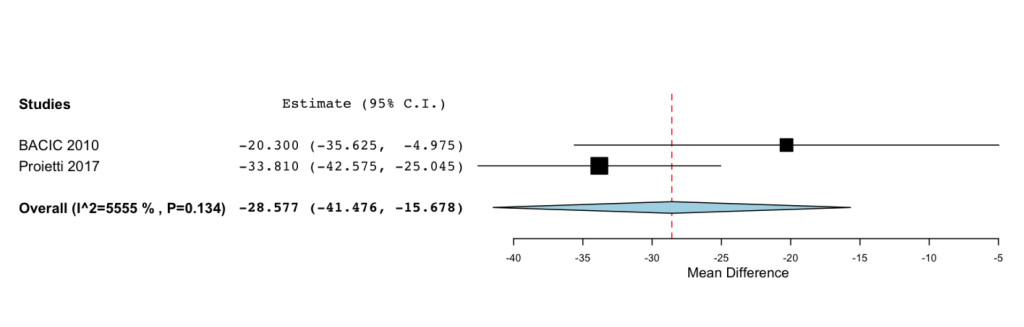
Figure 4. Forest plot showing effect sizes (mean difference (MD), 95% confidence interval (CI)) for Smptoms Scale in women undergoing chemotheraphy for breast cancer treated with IP6 (+MI).
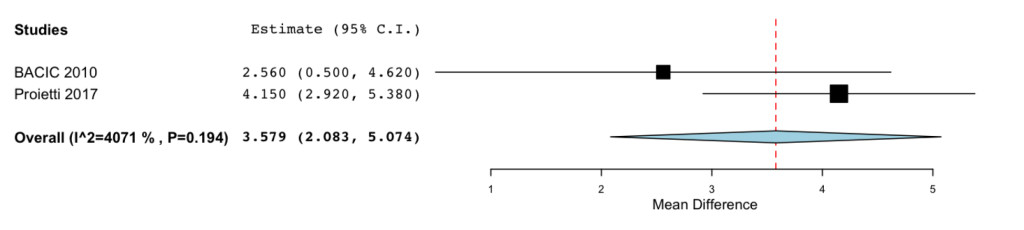
Figure 5. Forrest plot showing effect sizes (mean difference (MD), 95% confidence interval (CI)) for WBC in women undergoing chemotheraphy for breast cancer treated with IP6 (+MI).
Regarding platelet counts the analysis revealed considerable heterogeneity (Q(df=1)=7.870; Het. p-Value=0.005; I2=87.294). Nevertheless, it is worth to mention that in both studies, authors demonstrated that both treatments (either IP6 or IP6+MI) were able to prevent the drop of the platelet count.
Discussion
In the present review, we tried to highlight both new issues in the management of the oncological patient and new approaches in improving quality of life of patients undergoing chemotherapy.
In particular, literature data point at inositol(s), namely IP6 and MI, as an effective treatment able to improve patients QoL.
Indeed, literature data suggest that to improve cancer cure physicians have to improve cancer therapy effectives also by improving patients QoL, both during chemotherapy and afterwards.
MI as reaffirmed in a recent meta-analysis, is a well-known insulin sensitizer [13] nowadays hyperinsulinemia is considered a risk factor in cancer development. Indeed, in the majority of the tumors insulin regulated pathways are increased at both gene expression and activity of PI3K and Akt [14]. Evidence suggesting a positive role of Inositol (s) in cancer have been recently reviewed [4, 5, 15, 16]. Notably several authors have demonstrated that inositol phosphates and MI, in cancer cells, are able reduces PI3K expression (at both mRNA and protein level) [17] and Akt activation by inhibiting its phosphorylation [15, 18]. IP6 induce the impairment of the activity several signaling proteins such as: PI3K; PI3K-dependent activation of the tumor promoter induced AP-1; the phosphorylation-dependent activation of ERK [18]. Inhibition of PI3K activity, the protein kinase C (PKC) and the mitogen activated kinases (MAPK) have been documented by several studies both in vitro, [18-21] as well as in vivo, in particular, the in vivo studies were studies aiming to investigate inositol(s) chemo-preventive properties [22, 23].
In addition to the above described evidence that IP6 alone inhibits the growth of breast cancer cells, data by Tantivejkul et al., showed that IP6 synergistically acts with adriamycin or tamoxifen [24]. Noteworthy authors also manage to demonstrate that IP6 particularly effective when co-administerd with adriamycin or tamoxifen in ERα -negative cells and adriamycin resistant cell lines [24].
The clinical use of IP6+MI has been hampered by two main factors: bioavailability and palatability.
Human studies have demonstrated that dietary phytate is dephosphorylated during the digestion process by both plant phytases and phytases produced by human microbiota [25, 26].
Furthermore, several studies, aiming to investigate inositol phosphates solubility, have demonstrated that solubility in the stomach chyme negative correlates with the phosphorylation grade, i.e. the more phosphate group are attached to the inositol ring the less soluble and therefore bioavailable [27].
To solve this issue a transdermal gel has been used in the study by Proietti et al., indeed, instead of administering 1,4g of IP6 in powder, [9] researchers used a 5g of a 4% IP6 gel (200mg of IP6) [10].
In conclusion, literature data seams to demonstrate that IP6 and MI are effective in improving QoL of patients undergoing chemotherapy due to breast cancer.
References
- Siegel RL, Miller KD, Jemal A (2015) Cancer statistics. CA Cancer J Clin 65: 5–29.
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, et al. (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136: E359–386. [crossref]
- Bonadonna G, Valagussa P, Moliterni A, Zambetti M, et al. (1995) Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. N Engl J Med 332: 901–6.
- Bizzarri M, Dinicola S, Bevilacqua A, Cucina A (2016) Broad Spectrum Anticancer Activity of Myo-Inositol and Inositol Hexakisphosphate. Int J Endocrinol 5616807.
- Bizzarri M, Dinicola S, Cucina A (2017) Modulation of both Insulin Resistance and Cancer Growth by Inositol. Curr Pharm Des.
- Dinicola S, Minini M, Unfer V, Verna R, et al. (2017) Nutritional and Acquired Deficiencies in Inositol Bioavailability. Correlations with Metabolic Disorders. Int J Mol Sci 18.
- Moher D, Liberati A, Tetzlaff J, Altman DG, et al. (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med 3: e123–30.
- Collaboration TC (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 5.1.0 ed.
- Bacic I, Druzijanic N, Karlo R, Skific I, et al. (2010) Efficacy of IP6 + inositol in the treatment of breast cancer patients receiving chemotherapy: prospective, randomized, pilot clinical study. J Exp Clin Cancer Res 29: 12.
- Proietti S, Pasta V, Cucina A, Aragona C, et al. (2017) Inositol hexaphosphate (InsP6) as an effective topical treatment for patients receiving adjuvant chemotherapy after breast surgery. Eur Rev Med Pharmacol Sci 21: 43–50.
- Grases F, Simonet BM, Vucenik I, Prieto RM, et al. (2001) Absorption and excretion of orally administered inositol hexaphosphate (IP(6) or phytate) in humans. Biofactors 15(1): 53–61.
- Grases F, Isern B, Perelló J, Sanchis P, et al. (2006) Absorption of myo-inositol hexakisphosphate (InsP6) through the skin in humans. Pharmazie 61: 652.
- Unfer V, Facchinetti F2, Orrù B3, Giordani B3, Nestler J4 (2017) Myo-inositol effects in women with PCOS: a meta-analysis of randomized controlled trials. Endocr Connect 6: 647–658. [crossref]
- Pedrero JM, Carracedo DG, Pinto CM, Zapatero AH, Rodrigo JP, et al. (2005) Frequent genetic and biochemical alterations of the PI 3-K/AKT/PTEN pathway in head and neck squamous cell carcinoma. Int J Cancer 114: 242–248. [crossref]
- Dinicola S, Fabrizi G, Masiello MG, Proietti S, et al. (2011) Inositol induces mesenchymal-epithelial reversion in breast cancer cells through cytoskeleton rearrangement. Exp Cell Res 345: 37–50.
- Bizzarri M, Cucina A (2014) Tumor and the microenvironment: a chance to reframe the paradigm of carcinogenesis? Biomed Res Int 934038.
- Liu G, Song Y, Cui L, Wen Z, et al. (2015) Inositol hexaphosphate suppresses growth and induces apoptosis in HT-29 colorectal cancer cells in culture: PI3K/Akt pathway as a potential target. Int J Clin Exp Pathol 8: 1402–10.
- Huang C, Ma WY, Hecht SS, Dong Z (1997) Inositol hexaphosphate inhibits cell transformation and activator protein 1 activation by targeting phosphatidylinositol-3’ kinase. Cancer Res 57: 2873–8.
- Gu M, Roy S, Raina K, Agarwal C, et al. (2009) Inositol hexaphosphate suppresses growth and induces apoptosis in prostate carcinoma cells in culture and nude mouse xenograft: PI3K-Akt pathway as potential target. Cancer Res 69: 9465–72.
- Dong Z, Huang C, Ma WY (1999) PI-3 kinase in signal transduction, cell transformation, and as a target for chemoprevention of cancer. Anticancer Res 19: 3743–7.
- Bozsik A, Kökény S, Olah E (2007) Molecular mechanisms for the antitumor activity of inositol hexakisphosphate (IP6). Cancer Genomics Proteomics 4: 43–51.
- Gustafson AM, Soldi R, Anderlind C, Scholand MB, et al. (2010) Airway PI3K pathway activation is an early and reversible event in lung cancer development. Sci Transl Med 2: 26ra5.
- Han W, Gills JJ, Memmott RM, Lam S, et al. (2009) The chemopreventive agent myoinositol inhibits Akt and extracellular signal-regulated kinase in bronchial lesions from heavy smokers. Cancer Prev Res (Phila) 2: 370–6.
- Tantivejkul K, Vucenik I, Eiseman J, Shamsuddin AM (2003) Inositol hexaphosphate (IP6) enhances the anti-proliferative effects of adriamycin and tamoxifen in breast cancer. Breast Cancer Res Treat 79: 301–12.
- Sandberg AS, Andersson H (1988) Effect of dietary phytase on the digestion of phytate in the stomach and small intestine of humans. J Nutr 118: 469–473. [crossref]
- Schlemmer U, Jany KD, Berk A, Schulz E, et al. (2001) Degradation of phytate in the gut of pigs–pathway of gastro-intestinal inositol phosphate hydrolysis and enzymes involved. Arch Tierernahr 55: 255–80.
- Schlemmer U, Jany KD (2003) Proceedings of the Society of Nutritional Physiology; 57th conference. In: Breves G, editor: DLG-Verlag, Frankfurt (Main) 29.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, et al. (1999)The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85: 365–76.
- Fayers P, Aaronson NK, Bjordal K, Groenvold M, et al. (2001) EORTC QLQ-C30 Scoring Manual (3rd edition). 3rd ed. Brussels: European Organisation for Research and Treatment of Cancer.