Abstract
Bioactive silicate glass-based (PerioGlas®) has been previously used to enhance periodontal bone regeneration. However, the degradation of this glass in the body fluid generates a high pH (>8) which may enhance the growth of periodontopathic bacteria, such as Porphyromonas gingivalis (P. gingivalis) thereby inhibiting osteoblastic activity. The aim of this study was to: (i) develop a mixture of a phosphate and silicate glass to produce a more neutral pH environment where the alkaline pH arising from the bioactive silicate glass can be offset by the acidity of phosphate glass, (ii) whether the alkaline phosphatase enzyme (ALP) when added to the silicate/phosphate glass mixture can enzymatically hydrolyse the Q2 metaphosphate chains to release Q0 orthophosphate species that can be used in forming apatite and bone mineralization. For this purpose, nine compositions of bioactive silicate/phosphate glass-mixtures were prepared. The glass bioactivity was performed by immersing the prepared glass mixtures in ALP containing Tris buffer solution. The pH change in solutions was measured as a function of time. The glass mixtures degradation and apatite formation were investigated by 31P Solid and 31P Solution Nuclear Magnetic Resonance (NMR) spectroscopies. The results showed that the pH behaviour was modulated by immersing the glass-mixtures in buffered solutions. Solid and Solution NMR revealed that the terminal Q1 species belonging to the Q2-metaphosphate chains was hydrolysed by the ALP and converted into a Q0 orthophosphate species. In conclusion, the glass mixtures regulated the pH through its degradation stepwise on immersion. The output of the NMR spectra significantly supported the enzymatic degradation of glass mixtures with ALP enabling apatite precipitation for new bone formation. The concept of using silicate/phosphate glass mixtures with ALP is innovative and pioneering technology, suggesting its potentiality to develop new biomedical materials for different applications.
Keywords
Silicate glass, Phosphate glass, ALP activity, Apatite, Bone remineralisation
Introduction
Periodontal disease(s) can initiate irreversible damage to the underlying periodontal tissues as manifested by a loss of the attachment apparatus and alveolar bone resorption [1]. Bone loss is regarded as a serious clinical problem in dentistry (periodontology). The repair of damaged osseous tissues by using bioactive glass material as an alternative synthetic bone graft substance was a significant shift in perspective towards developing novel products for dental use [2]. A variety of biomaterials were developed for the application of both periodontal tissue regeneration and osseo-integration procedures in the defect site. These include materials such as barrier membrane, growth factors, bone graft material and combined procedures [3].
Bioactive silicate glass (PerioGlas®) has been used in the surgical treatment of periodontal bony defects as a synthetic bone graft substitute [4,5]. When PerioGlas® was implanted in vivo, an immediate exchange of ions occurs between the implanted glass and the surrounding environment. This resulted in the release of ions such as Ca2+ and PO43- which are then precipitated into a bone-like apatite on the glass surface [6-8]. The release of ions such as Na+ and Ca2+ may however, generate a rapid pH rise within the confines of the periodontal pocket, which was not previously considered in the published studies. One of the drawbacks of a high alkaline pH generated by PerioGlas® is the potential growth of P. gingivalis, which grows optimally at higher pH ≈ 8.3 [9]. Although this fact was widely acknowledged in the microbiological community working with these bacteria, it appears to have been completely overlooked within the dental materials community. Furthermore, this high alkaline pH inhibits the apatite formation by suppressing the ion exchange process of bioactive glass dissolution [10]. The high pH also retards bone formation through the inhibition of osteoblast activity and suppression of osteogenic differentiation/proliferation in the local biological environment [11].
Alkaline Phosphatase (ALP) has the unique feature of being involved in both pathogenic processes as well as in bone regenerative processes [12] and has been identified in gingival crevicular fluid (GCF), saliva and dental plaque bacteria [13]. Increased levels of ALP are also associated with the progression of periodontal disease from the healthy periodontium to a chronic periodontitis state indicating that this enzyme may be of diagnostic value in the diagnosis of periodontal diseases or at the very least a monitor of decreased/increased activity during periodontal treatment. It has been reported that there is a direct relationship between the elevated level of ALP and the severity of the periodontal disease [13,14]. ALP is released from active osteoblast, salivary glands, polymorphonuclear leukocytes, and periodontal bacteria [13,15]. To date, there is no biomaterial used in periodontal treatment that would account for the presence of elevated levels of ALP in periodontal bony defects. ALP is also known for its biological role in cleaving the P-O-P linkages in phosphate containing compounds. Grover et al., however demonstrated that Q0 orthophosphate species formed from the Q1-pyrophosphate species in presence of the ALP [16].
Therefore, the question to be addressed is whether ALP when added to a silicate/phosphate glass mixture can enzymatically hydrolyse the straight Q2-metaphosphate chain by cleaving Q1 terminal phosphate groups to release Q0 orthophosphate species. The Q0 orthophosphate species are the form of phosphate found in the mineral phase of natural bone, and therefore their formation may facilitate apatite precipitation and ultimately bone formation.
Materials and Methods
Glass Design and Synthesis
The design strategy of glass mixtures used in the present study is based on mixing one silicate glass (S-glass) composition with three compositions of phosphate glass (P-glass). Table 1 shows the compositions of the silicate glass and the three phosphate glasses in mole percent.
Table 1: Glass compositions used in the study (unit of mole%).
|
Mol% |
SiO2 | P2O5 | SrO | Na2O | K2O |
CaO |
| S-glass |
51.45 |
0 | 0 | 23.1 | 0 |
25.46 |
| P-glass (P1) |
0 |
55 | 30 | 0 | 15 |
0 |
| P-glass (P2) |
0 |
55 | 0 | 0 | 15 |
30 |
| P-glass (P3) |
0 |
55 | 10 | 0 | 15 |
20 |
Three ratios of P-glass to S-glass in the glass mixtures studied were used namely: 10/90, 25/75 and 50/50 by weight. Therefore, nine compositions of silicate/phosphate glass-mixtures were prepared. The 50/50 ratio utilized in the present study was the preferred ratio compared to the other two ratios based on experimental findings. The silicate glass has a coarse particle size (100-400 µm), whereas all three phosphate glasses have a fine particle size (<38 µm).
The ALP enzyme in Tris Buffer solution was made by adding 1.53 microliter of bovine ALP (Sigma-Aldrich) into a 100 ml Tris buffer solution. This addition was based on the calculated concentration of ALP enzyme identified in the GCF of periodontal bony defects [13]. The resulting solution was then stored in an incubator shaker (IKA® KS 4000i control, Germany) at a temperature of 37ºC and a pH 7.35 and subsequently used in the immersion test.
Immersion Protocol
0.15 g of the silicate/phosphate glass mixture was immersed in 10 ml of Tris Buffer to measure the pH change and 10 ml of ALP containing Tris Buffer to test the glass bioactivity. The suspended solutions were placed in plastic containers and these containers were stored in a shaking incubator at 37ºC for a specific period based on the range of immersion time points of the experiment. At the end of each immersion time point, the samples were removed from the incubator and the pH change in solutions measured with a pH meter (Oakton Instruments, Nijkerk, Netherlands). The suspended solution was then filtered using filter paper (Fisher Scientific) and the collected solid powders were placed in Petri dishes and stored in the drying cabinet at 37ºC overnight. The filtered solutions were kept in falcon tubes (Fisher Scientific) and stored in a fridge at 4ºC. The dried collected solid powders were characterized by 31P MAS-NMR, whilst the filtered solutions were characterized by 31P Solution NMR.
Magic Angle Spinning-Nuclear Magnetic Resonance (MAS-NMR)
The 31P MAS-NMR was performed using a Bruker probe and 600MHz Bruker spectrometer with a permanent magnetic field of 14.1 Tesla at the resonance frequency of 242.9 MHz. The spinning speed was 12 kHz and the solid sample was run for 16 minutes with 4 mm rotor. The recycle delay of 60s was used. The reference of the chemical shift was 85% H3PO4. The chemical species of 31P nucleus and glass structure were interpreted by the chemical shift and the intensity of the peaks. Both untreated and treated glass samples were investigated by this technique.
P Solution State NMR spectroscopy
The 31P solution NMR was run on a Bruker Avance III 400 MHz spectrometer. The frequency for 31P was 162.0 MHz. 32 scans acquired with a spectral width of 395.7 ppm (64102 Hz) were used prior to acquisition. The important NMR point being that ~10% Heavy Water D2O was added to the samples for the field/frequency lock. This analysis was performed by transferring 500 microliters of the aqueous samples required to be run into the NMR tubes (Wilmad® NMR tubes 5 mm diameter) together with 50 microliters of D2O using a Gilson pipette.
Results
Measurements of the pH trends of the three glass mixtures SP1, SP2 and SP3 together with their three ratios are demonstrated in Figure 1a, 1b and 1c respectively. The glass mixtures SP1, SP2 and SP3 with their three ratios (10/90, 50/50 and 25/75) exhibited smart modulation in pH behaviour in between the two undesirable extremes acidic and alkaline. The pH dropped initially with the phosphate glass. The phosphate glass reacts and dissolves faster and this is aided by a much smaller particle size than the silicate glass. In contrast the silicate glass results in an increase in pH.
It was clearly observed from Figure 1a, 1b and 1c that the initial pH drop can be manipulated by varying the ratios of glass mixtures 10/90, 50/50 and 25/75. The highest pH drop was with the ratio 10/90 where the phosphate glass was 90%, whereas the smaller pH drop was observed with the 25/75 ratio followed by 50/50 ratio, which regulated the pH around the physiological range.
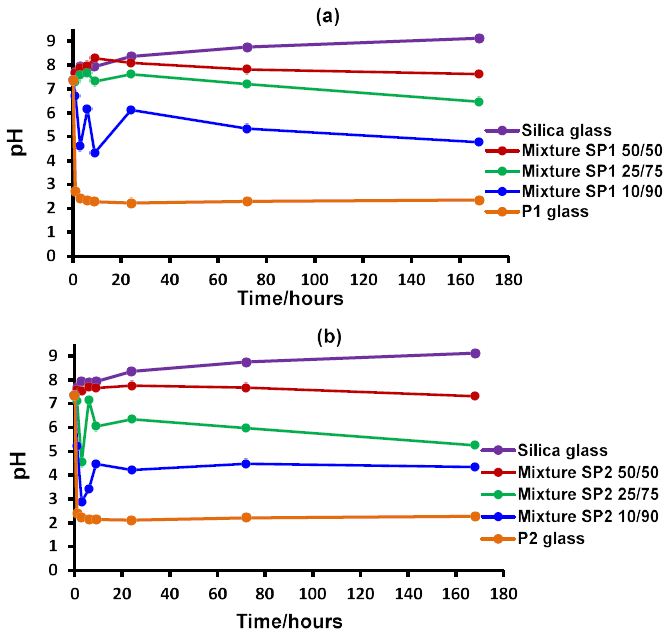
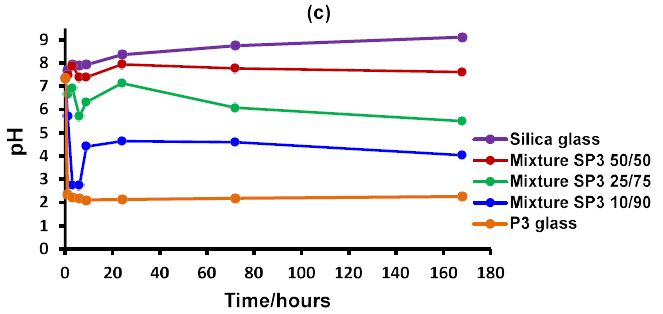
Figure 1: The pH behaviour in Tris buffer solution as a function of time of the three different ratios for each glass mixture together with discrete silicate and phosphate glasses: (a) glass mixture SP1; (b) glass mixture SP2 and (c) glass mixture SP3.
Therefore, based on the experimental pH data of glass mixtures provided above, the question of how to regulate this pH behaviour by varying the ratio of these glass mixtures should be explored in future studies.
Figure 2a and 2b shows the 31P MAS-NMR spectra of the solid material from the experimental glass mixture SP2 with the 50/50 ratio before and after ALP treatment as a function of time up to seven days. The signal at the position between (-26.4 ppm to -20 ppm) corresponds to the chemical shift of Q2-metaphosphate species [17,18]. The signal at the peak position between (-7.7 and -6.0 ppm) corresponds to the chemical shift of the Q1-phosphate species [17,18]. A minor signal around 2-3 ppm corresponds to the Q0-orthophosphate species [17,18]. Figure 2a shows the disappearance of Q2 peak at the expense of an increase in Q1 phosphates for different immersion times. However, there is also growth of a small Q0 calcium orthophosphate peak in the range 2-3 ppm although by seven days there is very little Q2 phosphates left.
On adding ALP, the spectra are similar, but there is an increased amount of Q0 calcium orthophosphate, and the peak is sharper and closer to the chemical shift for apatite of 2.9 ppm as shown in Figure 2b. Generally, the appearance of the Q0 signal in the sample immersed with ALP was sharper specifically in the seven-day immersion period.
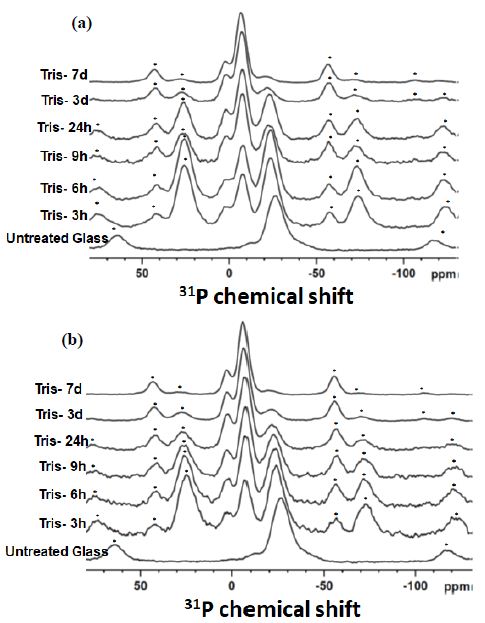
Figure 2: The 31P MAS-NMR spectra of the studied glass mixture SP2 with a 50/50 ratio before (a) and after (b) treatment with the ALP enzyme plotted for the immersion time points. Asterisks show spinning side bands; the spinning speed of 12 kHz was used for both (a) and (b).
Figure 3a and 3b shows the 31P solution NMR spectra of the experimental glass mixture SP2 with the 50/50 ratio before and after glass mixture treatment with ALP respectively as a function of time up to seven days. Three types of the signals were identified in the spectra. The signals with the chemical shift between (-25.2 ppm and -21.7 ppm) were assigned to the Q2-metaphosphate species [19]. The signal between (-9.8 ppm and -6.2 ppm) was assigned to the Q1-phosphate species [19]. Furthermore, the third signal with the position around 3 ppm corresponds to the Q0-orthophosphate species [19]. It can be observed according to the solution NMR spectra in Figure 3b that the higher amounts of the Q0-orthophosphate species were produced after treatment SP2 50/50 with the ALP enzyme compared to the spectra with no enzyme.
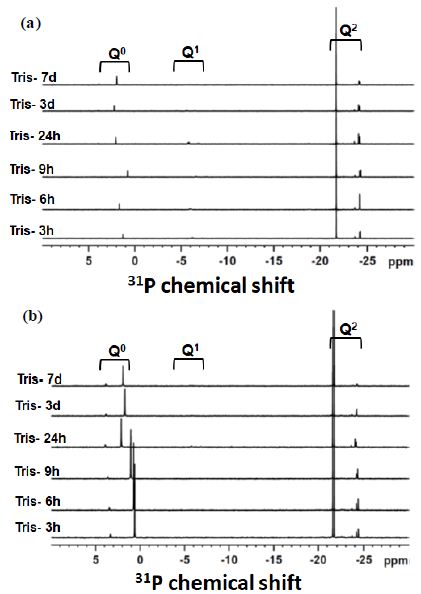
Figure 3: The 31P Solution NMR spectra of the solution remained after immersion of the glass mixture SP2 with a 50/50 ratio (a) without ALP treatment and (b) with ALP treatment. Immersion time points are indicated next to the spectra.
Figure 4 shows the 31P solution NMR integrals of the experimental glass mixture SP2 50/50 ratio with and without treatment with ALP as a function of time. With the glass mixture ratio without the enzyme treatment, the highest proportion of species released into the solution was Q2-metaphosphate (≈90%). Followed by the Q0-orthophosphate and the lowest proportion was the Q1 species. This was in good agreement with the study of Ahmed et al. [20]. In the presence of the enzyme, the proportion of the Q2 species decreased and the proportion of the Q0 species increased. There was also a reduction in the Q1 species after enzyme treatment.
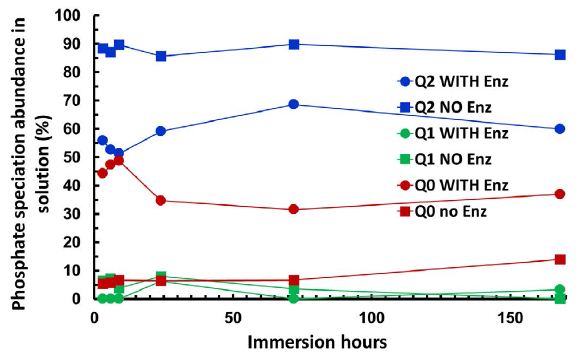
Figure 4: The integrals of three types of phosphate speciation Q2-Q1-Q0 seen in the 31P Solution NMR spectra of the studied glass mixture SP2 together with and without ALP treatment plotted as a function of time for 50/50 ratio. The error bar was estimated to be ± 0.02 for the data point.
Figure 5 compares the evolution of the decrease in Q2-metaphosphate species, with the increase in Q0-orthophosphate species and shows the decrease in both the Q2 and Q1 species together versus time. As can be observed from Figure 5 there were some differences between the proportion of the Q2 species lost (the blue points) and the proportion of the Q0 species gained (the orange points) as a function of time. In other words, the fraction of Q2 species did not match exactly with the Q0 species gained. However, there was a good match between the proportion of the (Q2 + Q1) lost (the grey points) and the proportion of the Q0 gained.
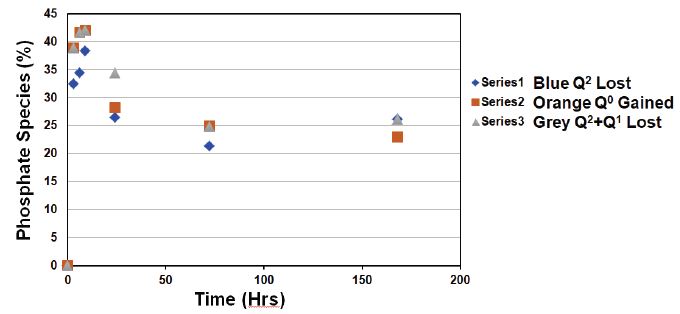
Figure 5: Illustrating the Q species lost or gained after treatment glass mixture SP2 50/50 ratio with ALP as a function of time.
Simple linear correlation coefficient and simple linear regression were used to describe the relationship between the Q species. Figure 6 demonstrates the correlation between the (Q2 + Q1) loss against the Q0 gained. This correlation is linear (R2 ≈ 0.97) and the slope is (0.986) which is close to (1.0). In other words, with the enzyme treatment the amount of (Q2 + Q1) loss was approximately equal to the amount of Q0 gained.
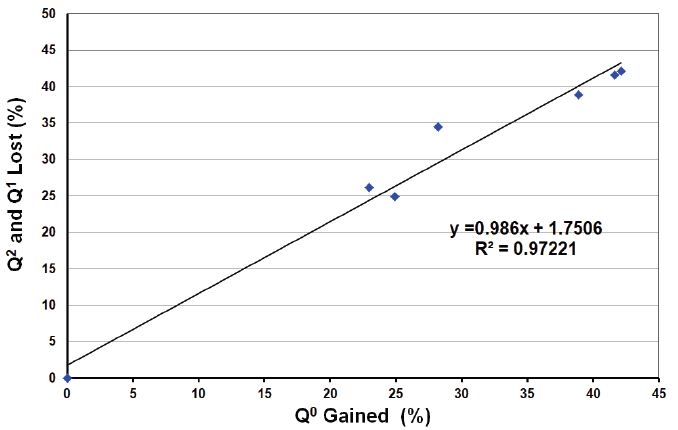
Figure 6: Illustrating the correlation of Q2 + Q1 loss after ALP treatment vs. the Q0 gained.
Discussion
The phosphate glass results in an acidic pH on dissolution, whereas the silicate glass produces an alkaline pH. The newly developed mixtures provide intermediate pH values. As the phosphate glass has a smaller particle size and degrades more rapidly there is a sharp reduction in pH initially followed by a slight rise in pH. The acidic pH produced by the phosphate glass accelerates the dissolution of the silicate glass. The data indicates how the pH can be adjusted by varying the proportions of the phosphate and silicate glasses. Enzymatic degradation of the P-O-P bond by the ALP is recognised for the Q1 pyrophosphate species. Grover et al. however demonstrated that Q0 formed from the Q1-pyrophosphate species in the presence of the ALP [16]. The solid state 31P MAS-NMR spectra showed that in the presence of ALP, the Q2-metaphosphate in the glass decreased much faster than when immersion without the ALP. Therefore, on adding ALP to the 50/50 mixture, the proportion of Q2 reduces and the proportion of Q0 increases, which is shown with the solution 31P NMR spectra. This suggests that the Q2 chains are hydrolysed by ALP where the Q2 species are being converted to Q0. The solution 31P NMR spectra showed several signals corresponding to each phosphate speciation. The results presented above reveal changes between three phosphate speciation(s) (Q2-Q1-Q0) in solution. For instance, the increase in the Q0 speciation can be identified as due to a strong increase in the Q0 signal at around 1-2 ppm in the presence of ALP.
The proposed mode of phosphate glass dissolution in solutions has been previously reported in a study by Ahmed et al. [20]. In the Ahmed study, the amount of the released phosphate species into the solution upon phosphate glass degradation has been investigated using ion chromatography. Based on the chromatogram data, Ahmed et al. [20] also stated that the phosphate species released into the solution after phosphate glass dissolution can be identified as the following: (i) a high proportion of the Q2-metaphosphate species which could be either linear chain or ring structure (unbranched) and the latter was suggested to be the predominant species in solution; followed by (ii) the Q0-orthophosphate species (PO43-); and (iii) a low proportion of the Q1-pyrophosphate species (dimer P2O7 4-). This would suggest that the Q1 observed from 31P NMR both in solution and the solid state are largely from Q1 end groups of Q2 chains. Moreover, the presence of both metaphosphate chains and rings in solution containing dissolution products of phosphate glasses in the 31P solution NMR were identified by Döhler et al. [19].
The presence of only a small fraction of Q1-phosphate was observed in the solution NMR spectra which would suggest that this fraction is more likely to be a terminal end of the Q2-metaphosphate chains rather than the pyrophosphate (dimer P2O7 4-)(each chain has two terminal Q1 phosphorus atoms). Additionally, some of the time points had no detectable fractions of Q1 even in the ALP-free samples in solution NMR spectra.
Based on the results presented above it is proposed that ALP cleaves the Q0 orthophosphate species from the terminal Q1 phosphate group of the Q2 metaphosphate linear chain. Therefore, the proportion of the Q1 species in solution is the lowest since it is no longer available in the solution. Each Q2 chain has two Q1 end groups and the Q1 end group is hydrolysed creating a new Q1 and a Q0 orthophosphate. This is shown schematically in Figure 7. The enzymatic hydrolysis continues until the chain is completely hydrolysed. However, a high proportion of the Q2 remained in solution NMR spectra even after seven days. This suggests that some Q2 species are not capable of being hydrolysed by ALP, although some are. We propose that Q2 chains are hydrolysed by ALP whereas Q2 cyclic phosphates contain no Q1 end groups and are therefore not capable of being enzymatically hydrolysed by ALP.
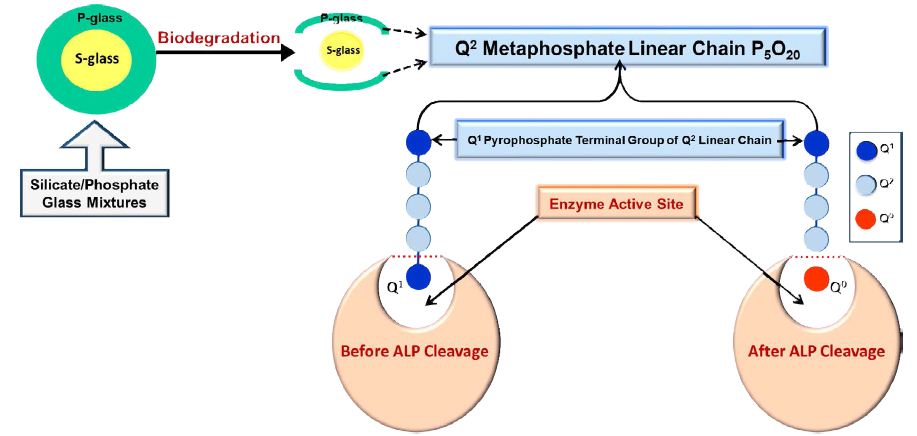
Figure 7: Schematic demonstration of the ALP enzyme activity in solution, describing the insertion of the Q2 metaphosphate linear chain in the active site of the ALP enzyme and the Q1 end group can be hydrolysed off to release Q0 orthophosphate species.
It would, however, be desirable to produce Q2 phosphate glasses that do not have any Q2 cyclic phosphates present but only linear chain phosphates. These glasses would then degrade to Q0 orthophosphate in vivo when ALP is present, such as in bone and in periodontal pockets and provide Q0 orthophosphate (PO43-) for apatite formation and bone mineralization.
Conclusion
Mixing a phosphate glass with silicate bioactive glasses can be used to avoid the undesirable low pH of phosphate glasses and the undesirable high pH generated by silica based bioactive glasses.
ALP hydrolyses the straight Q2 metaphosphate chain by cleaving Q1 terminal phosphate group to release Q0 orthophosphate (PO43-). The released Q0 orthophosphate ions are essential for apatite crystal formation. ALP should therefore be included in in vitro studies of the degradation of phosphate glasses to closely mimic their perceived in vivo behavior.
Acknowledgements
The Authors would like to thank Dr Harold Toms, (NMR facility manager) for his expert technical assistance. This study was part of a PhD funded by the Iraqi Ministry of Higher Education and Scientific Research.
References
- Socransky SS, Haffajee AD (1997) The nature of periodontal diseases. Annals of Periodontology 2: 3-10. [crossref]
- Aboelsaad NS, Soory M, Gadalla LMA, Ragab LI, Dunne S, et al. (2009) Effect of soft laser and bioactive glass on bone regeneration in the treatment of infra-bony defects (a clinical study). Lasers in Medical Science 24: 387-395. [crossref]
- Cho YD, Seol YJ, Lee YM, Rhyu IC, Ryoo HM, et al. (2017) An overview of biomaterials in periodontology and implant dentistry. Advances in Materials Science and Engineering 2017.
- Chacko NL, Abraham S, Rao HS, Sridhar N, Moon N et al. (2014) A clinical and radiographic evaluation of periodontal regenerative potential of PerioGlas®: a synthetic, resorbable material in treating periodontal infrabony defects. Journal of International Oral Health 6: 20. [crossref]
- Satyanarayanav K, Anuradha B, Srikanth G, Chandra P, Anupama T, et al. (2012) Clinical evaluation of intrabony defects in localized aggressive periodontitis patients with and without bioglass-an in-vivo study. Kathmandu Univ Med J 37: 11-15. [crossref]
- Wheeler D, Stokes K, Hoellrich R, Chamberland D, McLoughlin S (1998) Effect of bioactive glass particle size on osseous regeneration of cancellous defects. Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and the Australian Society for Biomaterials 41: 527-533. [crossref]
- Hench L. L, Polak JM (2002) Third-generation biomedical materials. Science 295: 1014-1017. [crossref]
- Profeta AC, G. M. Prucher (2015) Bioactive-glass in periodontal surgery and implant dentistry. Dental Materials Journal 34: 559-571. [crossref]
- Takahashi N, Schachtele C (1990) Effect of pH on the growth and proteolytic activity of Porphyromonas gingivalis and Bacteroides intermedius. Journal of Dental Research 69: 1266-1269. [crossref]
- Bingel L, Groh D, Karpukhina N, Brauer DS (2015) Influence of dissolution medium pH on ion release and apatite formation of Bioglass® 45S5. Materials Letters 143: 279-282,.
- L-E Monfoulet, P Becquart, D Marchat, K Vandamme, M Bourguignon, E Pacard et al., (2014) The pH in the microenvironment of human mesenchymal stem cells is a critical factor for optimal osteogenesis in tissue-engineered constructs. Tissue Engineering Part A 20: 1827-1840. [crossref]
- Groeneveld M, Bos T, Everts V, Beertsen W (1996) Cell‐bound and extracellular matrix‐associated alkaline phosphatase activity in rat periodontal ligament. Journal of Periodontal Research 31: 73-79. [crossref]
- Sanikop S, Patil S, Agrawal P (2012) Gingival crevicular fluid alkaline phosphatase as a potential diagnostic marker of periodontal disease. Journal of Indian Society of Periodontology 16: 513. [crossref]
- Bezerra Júnior AA, D. Pallos, J. R. Cortelli, and C. H. C. Saraceni (2010) Evaluation of organic and inorganic compounds in the saliva of patients with chronic periodontal disease. Revista Odonto Ciência 25: 234-238.
- Daltaban Ö, Saygun I, Bal B, Baloş K, Serdar M (2006) Gingival crevicular fluid alkaline phosphatase levels in postmenopausal women: effects of phase I periodontal treatment. Journal of Periodontology 77: 67-72. [crossref]
- Grover LM, Wright AJ, Gbureck U, Bolarinwa A, Song J, et al. (2013) The effect of amorphous pyrophosphate on calcium phosphate cement resorption and bone generation. Biomaterials 34: 6631-6637.
- Kirkpatrick RJ, Brow RK (1995) Nuclear magnetic resonance investigation of the structures of phosphate and phosphate-containing glasses: a review. Solid State Nuclear Magnetic Resonance 5: 9-21.
- MacKenzie KJ, Smith ME (2002) Multinuclear solid-state nuclear magnetic resonance of inorganic materials. Elsevier.
- Döhler F, Mandlule A, van Wüllen L, Friedrich M, Brauer DS (2015) 31 P NMR characterisation of phosphate fragments during dissolution of calcium sodium phosphate glasses. Journal of Materials Chemistry B 3: 1125-1134.
- Ahmed I, Lewis M, Nazhat S, Knowles J (2005) Quantification of anion and cation release from a range of ternary phosphate-based glasses with fixed 45 mol% P2O5. Journal of Biomaterials Applications 20: 65-80. [crossref]