Abstract
Soil transmitted helminth infections are still prevalent in many villages in Northern Ghana despite several interventions such as school-based deworming programmes. The study assessed the prevalence and socio-demographic characteristics of soil transmitted helminth (STH) infections among the people of Bunkpurugu in Northern Ghana. A sample size of 396 stool samples were collected from respondents and analyzed using the Kato-Katz technique (cellophane faecal thick smear) to determine the level of intestinal ova/eggs in collected stool samples. The overall prevalence for STH was 20.86%. The prevalence of hookworm was 19%, followed by Taenia at 1.4%, other soil transmitted helminths at 0.4%. There was a statistical relationship between sex and hookworm (P<0.001). Respondents between the ages of 11-15 years (OR 7.125, CI: 0.640-79.267) were seven times more likely to be tested positive for hookworm, those between 16-20 years (OR 5.55, CI: 0.541-56.907) were five times more likely to test positive for hookworm and those between the ages of 21-25 years (OR 10.87, CI: 1.058-111.757) were 10 times more likely to test positive for hookworm. Several helminths were recorded in this study with hookworm being the most predominant. The study therefore recommends that proper education and regular de-worming programmes should be organized in the study area.
Keywords
Helminths, Socio-demographic, Prevalence, Kato-Katz, Socio-demographic
Introduction
Soil transmitted helminth infections represent a significant burden on the developing world. The affected populations are typically the marginalized living in squalid conditions and representing the bottom billion people of the world [1]. In sub-Sahara Africa, approximately 250 million people are estimated to be infected with one or more helminths, thus polyparasitsm is a high possibility. Children of school going age usually bear the greatest brunt of helminthic infections. In these children, helminthic infections affect the cognitive development and hence accounting for regular school based de-worming programmes [2]. The World Health Organization estimates that approximately 1.5 billion people are infected with soil-transmitted helminths worldwide [3]. Hookworm infections represent the most prevalent among the soil transmitted helminth infections [4]. Hookworm disease caused by Ancylostoma duodenale and Necator americanus can cause iron deficiency and protein deficiency [5,6]. Most studies have neglected the role of socio-demographic factors and their influence on these helminthic infections. To properly target interventions to reduce the burden of these helminth infections, understanding of demographic factors such as age and sex distribution is very critical. This study therefore looked at the prevalence and socio-demographic determinants of helminth infections among the people of Bunkpurugu in the Northern part of Ghana.
Materials and Methods
Study Area
The study was conducted between April 2015 and September 2010 with the approval of the Ethics Committee of the Nugochi Memorial Institute for Medical Research, University of Ghana in the Bunkpurugu Constituency in the Bunkpurugu-Yunyoo District of the Northern Region of Ghana. The district occupies an area of about 70,383 square kilometers and is the largest region in Ghana in terms of land area.
Study Design and Sample Size
The sample size for this study was determined taking into consideration the estimated prevalence of the variable of interest, the acceptable margin of error (5%) and the desired level of confidence [95% Confidence level]. The sample size required was calculated as follows:
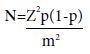
Where;
N = required sample size
Z = confidence level at 95%
p = estimated prevalence of Hookworm in the study district
m = margin of error at 5%.
At the end of the field work, the realized sample size was 396 for the parasitological studies.
Stool Sample Collection and Examination
Stool samples were collected over a five-month period from a random representative sample of 278 community members aged 10 years and above from the constituency.
Stool sample collection was done by recruited and trained research staff. All eligible participants were identified through a random selection of compounds/houses in selected communities using community registers. Stool sample containers were distributed to study participants in their homes a day before sample collection for them to provide samples the next morning. Fifty to sixty stool samples were collected in a day to allow for processing within 24 hours. Samples were appropriately labeled with the date of collection, identification and house numbers.
The collected samples were transported in ice-chests on ice parks to the Bimbagu Junior High School and processed the same day. Prepared slides were stored in a refrigerator and later transported to the parasitology laboratory of the Department of Animal Biology & Conservation Science, University of Ghana for examination by qualified laboratory personnel.
The Kato-Katz technique (cellophane faecal thick smear) was employed for the determination of the level of intestinal hookworm ova/eggs in collected stool samples [6]. The infestation was determined by microscopically examining 41.7mg of faecal material and systematically counting the eggs in the faecal specimens. To increase the visibility of the parasite eggs, the cellophane was soaked in a 3% methylene blue for 24 hours before usage. Quality control on 10% of the prepared slides (both positive and negative) was later done by an independent technologist.
Results and Discussion
Soil transmitted helminth infections are very common in resource-poor settings of the world where access to sanitation and water facilities is very problematic. This study affirms a study from Ethiopia which recorded that helminth infections account for the second most predominant causes of outpatient morbidity primarily due to lack of access to safe drinking water and improved sanitation facilities [7]. The major sources of drinking water for households in the District are borehole/pump/tube well, river/stream and unprotected well [8]. According to the 2010 Population and Housing Census, majority of households (80.5%) in the District do not have toilet facilities [8]. Most households resort to open defecation which leads to a high faecal load in the environment. The risk therefore of re-infection is very high even after deworming programmes [9]. The current study assessed and analysed various helminths and it was found that, hookworm was the predominant parasite (19%), followed by Taenia (1.4%) and other soil transmitted helminth infections (0.4%). No Ascaris and H. nana were recorded in this study. Soil transmitted helminth infections especially hookworm account for a global burden of 3.2 million disability adjusted life years [10,11]. It can be observed and emphasized that, out of the various helminths analysed, most of the respondents (19%) tested positive for hookworm eggs while the other helminths had less than 2% read among respondents. Despite the apparent importance of these helminths and the greater number of people infected, helminthic infections are classified as part of the Neglected Tropical Diseases and not part of the routinely diagnosed diseases in our public health system. These diseases are not less important as those of malaria, HIV/AIDS and tuberculosis (Figure 1 and Tables 1-6) [12].
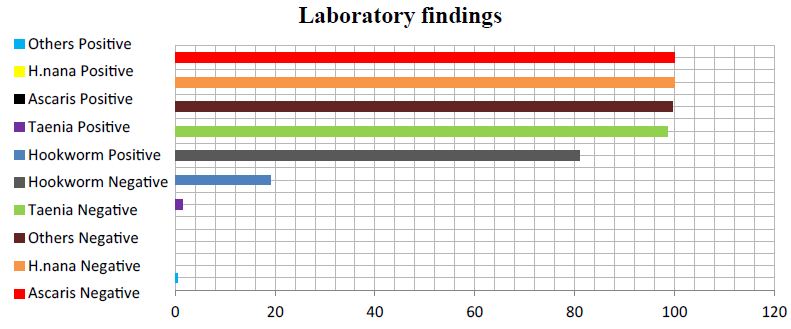
Figure 1: Frequency of various helminths from the study. From the table, 19% representing 53 respondents out of 278 tested positive for hookworm eggs, a percentage tested positive for Taenia while 99% tested negative. All 278 respondents representing 100% tested negative for H. nana. Another 100% tested negative for Ascaris while 1 respondent tested positive for other soil transmitted helminth infections.
Table 1: General Description of participants.
|
Variables |
Frequency |
Percentage |
| Gender | ||
| Males |
176 |
44 |
| Females |
220 |
56 |
| Total |
396 |
100 |
| Participants with laboratory read |
278 |
70 |
| Participants without laboratory read |
118 |
30 |
From the table, a little above half (56%) females and 44% were males. In all, 396 respondents participated in the study. Out of this, 278 respondents had laboratory read and 118 respondents had no laboratory read.
while 1 respondent tested positive for other soil transmitted helminth infections.
Table 2: Analysis for Participants with laboratory read (278).
|
Variable |
Frequency |
Percentage % |
|
Hookworm eggs |
||
| Positive |
53 |
19.1 |
| Negative |
225 |
80.9 |
| Total |
278 |
100 |
|
Taenia |
||
| Positive |
4 |
1.4 |
| Negative |
274 |
98.6 |
| Total |
278 |
100 |
|
Ascaris |
||
| Positive |
─ |
─ |
| Negative |
278 |
100 |
| Total |
278 |
100 |
|
H. nana |
||
| Positive |
─ |
─ |
| Negative |
278 |
100 |
| Total |
278 |
100 |
|
Others |
||
| Positive |
1 |
0.4 |
| Negative |
277 |
99.6 |
| Total |
278 |
100 |
From the table, 19% representing 53 respondents out of 278 tested positive for hookworm eggs, a percentage tested positive for Taenia while 99% tested negative. All 278 respondents representing 100% tested negative for H. nana. Another 100% tested negative for Ascaris while 1 respondent tested positive for other soil transmitted helminth.
Table 3: Prevalence of soil transmitted helminth infections.
|
Prevalence= Number of positive test X 100% Total number tested (278) |
||
|
Parasitic organism |
Positive Test |
Prevalence (%) |
| Hookworm |
53 |
19 |
| Taenia |
4 |
1.4 |
| Other soil transmitted helminths |
1 |
0.4 |
From the table, the prevalence of hookworm was 19%, Taenia was 1.4% and other soil transmitted helminth was 0.4%.
Table 4: Relationship between Hookworm, Taenia and socio-demographic characteristics of participants.
|
Hookworm |
|||
|
Attributes |
Yes; n (%) |
No; n (%) |
P-value |
|
Gender |
|||
| Male |
53 (30.1) |
123 (69.9) |
.001 |
| Female |
0 (0) |
102 (100) |
|
|
Age |
|||
| 5-10 |
8 (21.1) |
30 (78.9) |
.223 |
| 11-15 |
20 (20) |
80 (80) |
|
| 16-20 |
16 (14.7) |
93 (85.3) |
|
| 21-25 |
6 (31.6) |
13 (68.4) |
|
| 26+ |
3 (25) |
9 (75) |
|
|
Taenia |
|||
|
Gender |
|||
| Male |
4 (2.3) |
172 (97.7) |
.063 |
| Female |
0 (0) |
102 (100) |
|
|
Age |
|||
| 5-10 |
2 (5.3) |
36 (94.7) |
.093 |
| 11-15 |
2 (2) |
98 (98) |
|
| 16-20 |
0 (0) |
109 (100) |
|
| 21-25 |
0 (0) |
19 (100) |
|
| 26+ |
4 (1.4) |
274 (98.6) |
|
A bivariate analysis was conducted to ascertain the association between the outcome variable and various independent variables. The results indicate that gender (P<0.000) had a relationship with whether respondents will test positive or negative for hookworm. Age (P=0.223) had no relationship with been tested with hookworm. It was also found that, gender (P=0.063) and age (P=0.093) had no relationship as to whether a respondent will test positive or negative for Taenia.
Table 5: Odds ratio between Hookworm and age distribution of participants.
|
Hookworm Adjusted Odds Ratio |
95% CI |
||
|
Age |
|||
| 5-10 |
Ref |
||
| 11-15 |
7.125 |
.640 |
79.267 |
| 16-20 |
5.550 |
.541 |
56.907 |
| 21-25 |
10.875 |
1.058 |
111.757 |
| 26+ |
4.000 |
.329 |
48.656 |
In order to control for confounders and determine the predictors of hookworm, a logistic regression was calculated. The model took into consideration all significant variables at the simple logistic regression level, of which age was the only significant variable. The result indicates that, respondents between the ages of 11-15 years (OR 7.125, CI: 0.640-79.267) were seven times more likely to be tested with hookworm, those who were between 16-20 years (OR 5.55, CI: 0.541-56.907) were five times more likely to be tested with hookworm and finally those between the ages of 21-25 years (OR 10.87, CI: 1.058-111.757) were 10 times more likely to be tested with hookworm.
Table 6: Gender and Age distribution of Hookworm eggs. Laboratory analysis on hookworm only (n=53).
|
Gender |
Hookworm eggs |
Total |
|
|
Low counts |
High counts |
||
| Male |
13 |
13 |
26 |
| Female |
27 |
0 |
27 |
| Total |
40 |
13 |
53 |
| Age | |||
| 5-10 |
2 |
6 |
8 |
| 11-15 |
15 |
5 |
20 |
| 16-20 |
15 |
1 |
16 |
| 21-25 |
5 |
1 |
6 |
| 26+ |
3 |
0 |
3 |
| Total |
40 |
13 |
53 |
Table 5 shows the gender distribution and the count of hookworm eggs for respondents who tested positive for hookworm. Out 0f 53 respondents, 27 females had low count of hookworm eggs, 13 males had high counts and the other 13 had low hookworm counts. On the age distribution, 15 respondents who were between the ages 11-15 years had low counts of hookworm, another 15 respondents between the ages of 16-20 years had low counts and 6 respondents between the ages of 5-10 years had high counts of hookworm eggs.
The overall prevalence of soil transmitted helminth infection found in this study was 20.86%. This is particularly corroborated by a study in Ethiopia who also found the prevalence of STH to be 20.9% [13]. Similarly high prevalence was recorded in Uttar Pradesh in India [14]. High prevalence means that interventions such as school-based deworming programmes have failed to yield the needed result.
The results of the study demonstrated that hookworm infection in the study area varies with demographic factors such as age and sex. The study established a statistical relationship between gender and hookworm (P<0.001). Males were more likely to test positive for hookworm than females. A study in Thailand also found similar results [3]. This could be attributed to the high-risk behaviours of males. With respect to hookworm and age, respondents between the ages of 11-15 years (OR 7.125, CI: 0.640-79.267) were seven times more likely to be tested with hookworm, those who were between 16-20 years (OR 5.55, CI: 0.541-56.907) were five times more likely to be tested with hookworm while those between the ages of 21-25 years (OR 10.87, CI: 1.058-111.757) were over seven times more likely to be tested with hookworm. Similar studies have also shown strong correlations of hookworm infections with age [15,16].
People become infected with hookworm when they get into contact with the soil contaminated with the hookworm larvae. Hookworm infection is also common among people who walk barefoot in especially places with warm climate and compromised sanitation. This is exactly the situation in the study area. Access to improved sanitation in the area is difficult and people resort to open defecation [17].
Conclusions and Recommendations
The study recorded an overall prevalence of STH was 20.86%. The predominant helminth was hookworm (19%). No H. nana and Ascaris were recorded in the study. This could be due to the low sensitivity of technique used. Demographic variables such as age and gender influenced whether a person became infected with hookworm or not.
Acknowledgement
The authors want to sincerely thank all the participating schools and communities for their immense support during this survey.
References
- Boatin BA, Basanez MG, Prichard RK, Awadzi K, Barakat RM, et al. (2012) A Research Agenda for Helminth Diseases of Humans: Towards Control and Elimination. PLoS Negl Trop Dis 6: 1547. [crossref]
- Addo HO, Ako-Nnubeng IT (2014) The Impact of the School Based Deworming Program on Education in the Kwahu West Municipality of Ghana. Env and Earth Sci
- Punsawad C, Phasuk N, Bunratsami S, Thongtup K, Siripakonuaong N, et al. (2017) Prevalence of intestinal parasitic infection and associated risk factors among village health volunteers in rural communities of Southern Thailand. BMC Public Health 17: 564. [crossref]
- Forrer A, Vounatsou P, Sayasone S, Vonghachack Y, Bouakhasith D, et al. (2015) Risk Profiling of Hookworm infection and Intensity in Southern Lao People’s Democratic Republic Using Bayesian Models. PLoS Negl Trop Dis 9: 0003486. [crossref]
- Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, et al. (2006) Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 367: 1521-32. [crossref]
- Katz N, Chaves A, Pellegrino JA (1972) Simple Device for the quantitative stool thick-smear technique in Schistosoma mansoni. Rev Inst Med Trop Sao Paulo 14: 397-400. [crossref]
- Alemu A, Atnafu A, Addis Z, Shiferaw Y, Teklu T, et al. (2011) Soil transmitted helminths and Schistosoma mansoni infections among school children in Zarima town, northwest Ethiopia. BMC Infect Dis 11: 189. [crossref]
- Ghana Statistical Service, Population and Housing Census, 2010.
- Jia TW, Melville S, Utzinger J, King CH, Zhou XN (2012) Soil-transmitted helminth re-infection after drug treatment: a systematic review and meta-analysis. PLoS Negl Trop Dis 6: 1621. [crossref]
- Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, et al. (2012) Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2197-2223. [crossref]
- Pullan RL, Smith JL, Jasrasaria R, Brooker SJ (2014) Global numbers of infections and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors 7:37. [crossref]
- Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE et al. (2007) Control of Neglected Tropical Diseases. N Engl J Med 357: 1018-1027. [crossref]
- Shiferaw MB, Mengistu AD (2015) Helminthiasis: Hookworm Infection Remains a Public Health Problem in Dera District, South Gondar, Ethiopia. PLoS ONE
- Ganguly S, Barkataki S, Karmakar S, Sanga P, Boopathi K, et al. (2017) High Prevalence of soil-transmitted helminth infections among primary school children, Uttar Pradesh, India. Infect Dis Poverty 6: 139. [crossref]
- Gandhi NS, Jizhang C, Khoshnood K, Fuying X, Shanwen L, et al. (2001) Epidemiology of Necator americanus hookworm infections in Xiulongkan village, Hainan Province, China: high prevalence and intensity among middle-aged and elderly residents. J Parasitol 87: 739-743. [crossref]
- Jardim-Botelho A, Brooker S, Geiger SM, Fleming F, Souza Lopes AC, et al. (2008) Age patterns in undernutrition and helminth infection in a rural area of Brazil: associations with ascariasis and hookworm. Trop Med Int Health 13: 458-467. [crossref]
- Halpenny CM, Paller C, Koski KG, Valdes VE, Scott ME (2013) Regional, household and individual factors that influence soil transmitted helminth reinfection dynamics in preschool children from rural indigenous Panama. PLoS Negl Trop Dis 7: 2070. [crossref]