Abstract
Aim: To study whether a non-surgical therapy such as scaling and root planing (SRP) will help in improving the glycaemic control in type 2 diabetes (T2D) patients as assessed by Glycosylated haemoglobin (HbA1c) measured at baseline and during the follow-up dental examinations in a diabetes hospital in Southern India.
Methods: In this retrospective study among T2D patients, the intervention group underwent SRP in addition to conventional treatment for hyperglycaemia, the control group had only conventional treatment. Both groups had mild to moderate Periodontal Disease (PD) at baseline. Glycaemic variations, change in HbA1c were assessed between 4 and 6 months. Impact of baseline characteristics and SRP on follow-up HbA1c was assessed using multiple logistic regression analysis.
Results: Out of the 1164 patients identified, 319 were selected, 124 patients in the control group and 135 in the intervention group satisfied the criteria for analysis. At baseline, gender distribution, diabetes duration, body mass index and mean HbA1c were similar in these groups. The intervention group was younger (p = 0.02), higher percentage of them were on oral hypoglycaemic agents (p = 0.006).
At follow-up, only the intervention group showed a significant reduction in HbA1c (9.0% (75 mmol/mol) to 8.0% (64 mmol/mol), p<0.0001), while no change was seen in the control group (8.7% (72 mmol/mol) to 8.8% (73 mmol/mol)). Importantly with intervention 52.6% shifted to optimal level of HbA1c, while 52.3% of the control group had uncontrolled diabetes. Improvement in HbA1c was inversely associated with baseline HbA1c, duration of diabetes and treatment with only oral hypoglycaemic agents (OHA) versus OHA plus insulin. Intervention with SRP was independently and significantly associated with improvement in HbA1c (≤7.5%, 59 mmol/mol) [odds ratio (OR), 95% confidence interval (CI) 3.390 (1.786-6.434), p<0.0001].
Conclusion SRP, a simple and practical procedure had independent, significant beneficial effect on glycaemic control among Asian Indian T2D patients with PD.
Keywords
Type 2 diabetes, Periodontitis, Scaling and root planing, Glycaemic control, Glycosylated haemoglobin
Introduction
Diabetes is a major healthcare challenge both in developed and in developing countries. India has a large number with diabetes (72 million) and more than 90% of them have type 2 diabetes (T2D) [1]. Diabetes poses a healthcare burden not only because of the chronic requirement for the management of hyperglycaemia but also due to the associated micro and macro vascular risk factors and disorders [1,2]. Persistent hyperglycaemia can lead to several complications which include periodontal disease (PD), known to be a major complication of diabetes [3,4]. PD is caused due to exogenous bacterial infection and the resultant host response to bacterial challenge. The increased inflammatory response destroys both the endogenous bacteria and also releases cytokines that causes destruction of periodontal tissues [5]. There is emerging evidence to support the existence of a bidirectional relationship between diabetes and PD [6-9] with diabetes increasing the risk for PD and periodontal inflammation negatively affecting glycemic control [10]. It is also suggested that periodontitis may be a risk factor itself for other diabetes complications [11]. Due to the non-uniformity of the methodology, regions, age groups, the presence of habits such as tobacco use and awareness about oral hygiene it is not possible to derive a definite rate of prevalence of periodontitis in India [12,13].
We investigated in this study the effect of a non-surgical periodontal therapy, scaling and root planing (SRP) on metabolic control among T2D patients. There are only a few studies from India on the beneficial effect of SRP on glycaemic control [14-16]. Moreover, studies in large numbers and also with a comparative group are limited.
The aim was to analyse the change in glycaemic control as assessed by the HbA1c values measured at the time of baseline dental examination and during the follow-up after SRP in comparison with a control group without SRP.
Methods and Materials
Patient Selection
This was a retrospective study among T2D patients who were referred to the dental department of Dr.A.Ramachandran’s Diabetes Hospitals, Chennai, India. It included a study group who underwent periodontal therapy and a control group that only had the usual care for diabetes during the study period. The selection of patients for the analysis is shown in the flow diagram (Figure 1). Patients with diagnosis of PD and had follow-up data between 4 and 6 months of their initial visit were included. For this, medical records of T2D patients, both men and women of age 25-65 years followed-up during June 2018 and December 2019 were selected. The reasons for exclusion were unwillingness to undergo a mechanical treatment, inability to report for follow-up within the prescribed time period or having had treatment with antibiotic or anti-inflammatory drugs. Patients with habit of smoking, pan or tobacco chewing were also excluded.
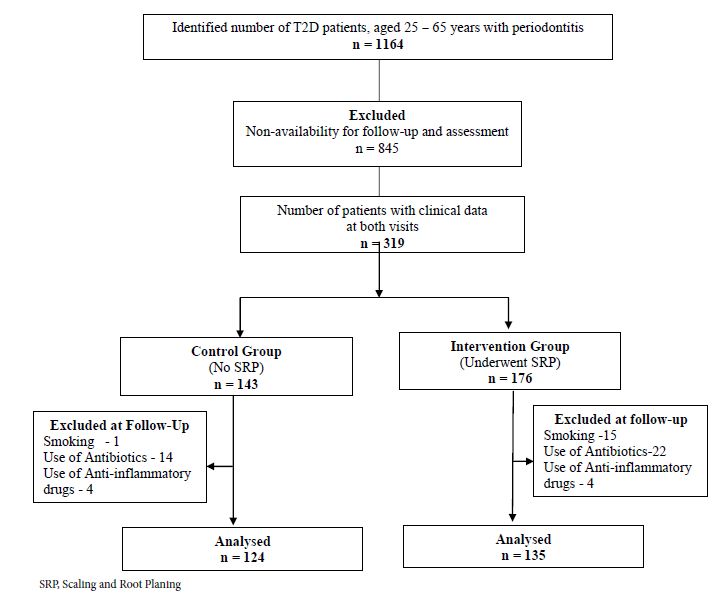
Figure 1: Flowchart showing the selection of study participants and group allocation.
A written informed consent was obtained from the patients prior to inclusion in the study to use their clinical data for research purposes without disclosing their identity. The study was approved by the Ethics Committee of the India Diabetes Research Foundation and Dr. A. Ramachandran’s Diabetes Hospitals, Chennai.
Clinical and Dental Assessment
Details on patient’s treatment history including advice on diet and physical activity were recorded. Body mass index (BMI, kg/m2) was calculated. Initial diagnosis of diabetes was made using the World Health Organisation criteria with a fasting plasma glucose (FPG) level ≥ 126 mg/dl (7.0 mmol/l) and/or 2-h post-load glucose level ≥ 200 mg/dl (11.1 mmol/l) [17]. We recorded HbA1c levels at the baseline and during the follow-up visits. HbA1c was measured by immunoturbidimetry method using TINA-QUANT II (Roche Diagnostics Corporation, Germany).
Presence of mild to moderate chronic periodontitis was diagnosed with a probing depth of >5 mm and clinical attachment loss (CAL) of >3 mm and radiographic evidence of 30 to 50% bone loss.
Study Groups
Patients chosen for the intervention group underwent SRP after the initial diagnosis of PD. The control group did not undergo SRP but received conventional treatment for glycaemia. Both groups reported for follow-up to the dental department between 4 and 6 months of the baseline visit.
Statistical Analyses
Severity of glycaemia is shown in terms of mean HbA1c values categorized as mild (HbA1c ≤7.5% (59mmol/mol)), moderate (HbA1c 7.6(60 mmol/mol)-8.5% (69 mmol/mol)) and severe (HbA1c >8.5% (>69 mmol/mol)) glycaemia. Comparisons between the baseline and follow-up values in the total group and in the 3 categories of glycaemia were made. The impact of intervention (SRP) on the mean HbA1c values and also in the 3 categories of HbA1c was compared with the respective values in the control group during the follow-up.
Data are presented as mean ± SD for continuous variables with a normal distribution, as median (interquartile range) for skewed variables and as frequency (%) for categorical variables. Intergroup differences were tested using independent sample‘t’ test and chi-square test for continuous and categorical variables respectively. For skewed variables Mann-Whitney U test was used. A multiple logistic regression analysis (MLR) (enter method) was done to assess the impact of baseline variables and SRP versus conventional treatment on the control of HbA1c at follow-up. The dependent variable was HbA1c of ≤7.5% (59 mmol/mol) versus >7.5% (>59 mmol/mol) at follow-up. Independent variables used were age, BMI, duration of diabetes, baseline HbA1c (as continuous variables), gender (reference: female), treatment of diabetes (only oral hypoglycemic agents (OHA) versus insulin plus OHA (reference)) and groups (SRP versus no SRP (reference)).
All statistical analyses were done using IBM SPSS (version 21.0). A value of p < 0.05 was considered as statistically significant.
Results
Among the total of 1164 records identified, patients from outstation (n = 845) were excluded because of their inability to report for follow-up visit during the study period. Details of 176 patients who underwent SRP (Intervention group) and 143 patients who did not undergo SRP (Control group) were included in the study. Patients in both study groups reported for the clinical follow-up between 4 and 6 months of their baseline visit. Among them, 19 patients in the control group and 41 patients in the intervention group were excluded due to the requirement for treatment with antibiotic or anti-inflammatory drugs or because of smoking habits. For the final analysis, 124 patients in the control group and 135 patients in the intervention group were included (Figure 1).
The baseline characteristics of the study participants in the control and intervention groups are shown in Table 1. The gender distribution, duration of diabetes, BMI and mean HbA1c values were similar in both groups. Higher percentage (p<0.0001) in the intervention group had calculi and/or stains. The intervention group was younger (p = 0.02), and a higher percentage of them was on treatment with OHA for diabetes (p = 0.006). Only a small percentage was on lifestyle modification, more so in the control group (p = 0.02).
Table 1: Baseline characteristics of the Control and Intervention groups.
|
Variables |
Control n = 124 |
Intervention n = 135 |
| Gender | ||
| Male, n (%) |
83 (66.9) |
94 (69.6) |
| Female, n (%) |
41 (33.1) |
41 (30.4) |
| Age (years) mean±SD |
55.4 ± 6.5* |
53.4 ± 7.2 |
| BMI (kg/m2) mean±SD |
27.4 ± 4.2 |
27.1 ± 5.4 |
| Duration of Diabetes (months) median, IQR |
170 (96-252) |
133 (90-224) |
| Treatment | ||
| OHA, n (%) |
60 (48.4) |
88 (65.1) # |
| Insulin ± OHA, n (%) |
52 (41.9) |
43 (31.9) |
| Diet & Exercise, n (%) |
12 (9.7)$ |
4 (3.0) |
*p = 0.02 (‘t’ test), #p = 0.006, $p = 0.02 (Chi-square test).
BMI: Body Mass Index; OHA: Oral Hypoglycemic Agent.
Figure 2 shows the changes in the mean HbA1c values at the baseline and follow-up in the study groups. In the control group, both values were similar, whereas in the intervention group the mean value had decreased significantly at the follow-up (p<0.0001). As mentioned above, the mean baseline HbA1c value was similar in both groups. At follow-up, the control group had a higher value when compared with the intervention group (p<0.0001).
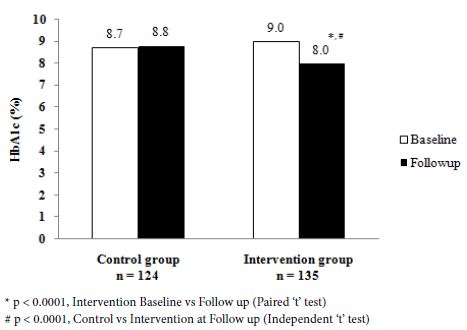
Figure 2: Mean HbA1c (%) at baseline and at follow up.
Figure 3 shows the distribution in percentage in the categories of HbA1c in the control and intervention groups. At the baseline (Panel A), the maximum number of patients in both groups were in the highest category (>8.5% (69 mmol/mol)) of HbA1c. At follow-up (Panel B), it was observed that with intervention a larger percentage had shifted to the optimal level of HbA1c (52.6%, p<0.0002). In contrast, a larger percentage of the control group was in the uncontrolled category (53.2%, p<0.0001).
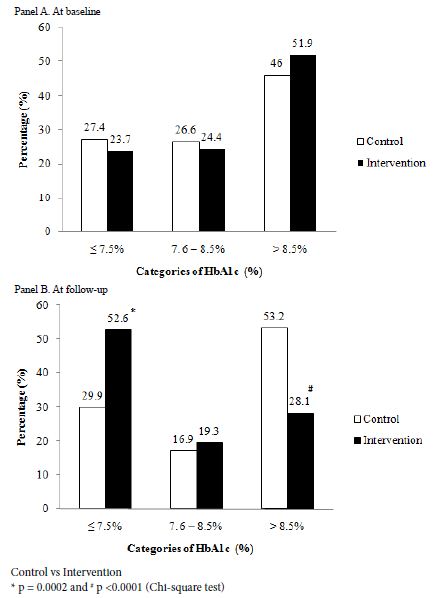
Figure 3: Distribution in Percentage in the categories of HbA1c
In the second category of HbA1c (7.6% (60 mmol/mol)-8.5% (69 mmol/mol)) there was no significant difference between the values at baseline and follow-up in either group.
The multiple logistic regression analysis showed that age, gender and BMI did not have significant association with the outcome. The duration of diabetes, baseline HbA1c and treatment with only OHA had inverse association with good control of HbA1c. Treatment with SRP had a significant influence on the glycaemic outcome independent of the above parameters [odds ratio (OR), 95% confidence interval (CI) 3.390 (1.786-6.434), p<0.0001] (Table 2).
Table 2: Variables associated with the glycaemic outcome (HbA1c) – results of the multiple logistic regression analysis.
|
Variables |
β Constant (SE) |
OR (95% CI) |
p value |
| Age (years) |
0.044 (0.026) |
1.045 (0.993-1.100) |
0.091 |
| Gender (Male) |
0.648 (0.361) |
1.911 (0.942-3.875) |
0.073 |
| BMI (kg/m2) |
-0.014 (0.036) |
0.986 (0.920-1.057) |
0.699 |
| Duration of diabetes (months) |
-0.004 (0.002) |
0.996 (0.992-1.000) |
0.035 |
| Baseline HbA1c (%) |
-0.534 (0.108) |
0.586 (0.474-0.724) |
<0.0001 |
| Treatment of Diabetes (OHA) |
-0.926 (0.382) |
0.396 (0.187-0.837) |
0.015 |
| Group (Intervention) |
1.221 (0.327) |
3.390 (1.786-6.434) |
<0.0001 |
Dependent variable: HbA1c of ≤ 7.5% (59 mmol/mol) versus >7.5% (59 mmol/mol) at follow-up.
Independent variables used in the equation were age, BMI, duration of diabetes, baseline HbA1c (as continuous variables), gender (reference: female), treatment of diabetes (only oral hypoglycemic agents (OHA) versus insulin plus OHA (reference)) and groups (SRP versus no SRP (reference)).
OR: Odds Ratio; CI: Confidence Interval; BMI: Body Mass Index; OHA: Oral Hypoglycemic Agents.
Treatment with SRP showed a definite additive effect on the conventional treatment for diabetes in patients who also had PD.
Discussion
In this study, the important observation was that mechanical treatment of PD with SRP had facilitated improvement of glycaemia in diabetes patients during the follow-up assessment between 4 to 6 months. In comparison with the control group, treated with the conventional methods including regular clinical follow-up, better glycaemic outcome was seen with SRP even among the patients who had severe glycaemia. At follow-up, majority of the patients in the intervention group showed optimal control of glycaemia when compared to the control group (52.6% versus 29.9%, p = 0.0002). The MLR showed that treatment with SRP had an independent impact on the improvement of glycaemic outcome [OR (95% CI): 3.390 (1.786-6.434), p<0.0001]. Persons with uncontrolled diabetes and also required combined OHA and insulin treatment showed better impact with SRP as indicated by the inverse significant association with the outcome.
Previous studies have also reported the beneficial effects on diabetes control as measured by the HbA1c levels following non-surgical periodontal treatment [14-16,18]. While the first line of treatment for glycaemic control is lifestyle modification, use of OAD and /or insulin and adjunctive therapy for PD such as SRP is shown to result in better glycaemic outcome [14,18]. SRP removes the causative factors such as plaque and calculi which result in inflammation and improves the glycaemic control, also preventing its further accumulation [19]. Improved HbA1c can be also attributed to diminished gingivitis. Number of studies in India [14-16] and in several other countries [18,20] had reported that periodontal therapy is associated with reduction of infection and inflammation facilitating metabolic control of diabetes. However, some studies did not have comparative data from control groups [14,21]. A study by Stewart et al. [18] showed improvement in 17.1% in 10 months versus 6.7% in matched control groups.
Another study, with a controlled study design in India had reported outcomes similar to our findings [15]. However, the study group comprised of only 45 T2D patients [15]. A larger study in India by Sunder et al, in 266 T2D patients with a post treatment HbA1c level of 8.4 (68 mmol/mol) ± 1.9% showed a significant reduction following SRP in a follow-up period of 6 months [14]. The baseline HbA1c, mean age and inclusion criteria were similar to our study design, but there was no control group included.
Some studies had shown an effect of mechanical treatment as observed in our study and few others showed a combined effect of mechanical and antibiotic / anti-inflammatory treatment for PD on glycaemic control [22].
While many studies showed enhanced benefit of SRP as an adjunctive therapy for PD in glycaemic control, a few studies did not support this observation [23-27].
Our study has shown that in addition to the conventional therapy for hyperglycaemia, mechanical therapy such as SRP has a beneficial role even among T2D patients with severe hyperglycaemia. This procedure is simple and practical with minimal discomfort to the patients. Periodic dental check-up and application of such adjunctive therapy should become a part of diabetes management.
Declarations
Funding
The study was funded by India Diabetes Research Foundation, Chennai.
Conflict of Interest
None
Ethics Approval
The study was approved by the Ethics Committee of the India Diabetes Research Foundation and Dr. A. Ramachandran’s Diabetes Hospitals.
Authors’ Contributions
RV, CS, Arun R, AN, AR contributed to the study design. RY, A Rajeswari coordinated in data collection. AR, CS, KS and PS contributed to analyses and drafted the manuscript. All authors have reviewed the manuscript with critical input and approved the final draft of the manuscript.
Acknowledgements
We are grateful to all the patients for having consented to utilise their medical records for the purpose of research analysis. The support rendered by the department of dental care of Dr.A.Ramachandran’s Diabetes Hospitals, Chennai is greatly acknowledged.
Abbreviations
BMI: Body Mass Index
CAL: Clinical Attachment Loss
CI: Confidence Interval
FPG: Fasting Plasma Glucose
HbA1c: Glycosylated Haemoglobin
MLR: Multiple Logistic Regression
OHA: Oral Hypoglycaemic Agents
OR: Odds Ratio
PD: Periodontal Disease
SRP: Scaling and Root Planing
T2D: Type 2 Diabetes
References
- International Diabetes Federation. IDF Diabetes Atlas, 9th edn. Brussels, Belgium: 2019. Available at: http://www.diabetesatlas.org. Last accessed on 11th March 2020.
- Ramachandran A, Ma RC, Snehalatha C (2010) Diabetes in Asia. Lancet 375: 408-418. [crossref]
- Jones JA, Miller DR, Wehler CJ, Rich SE, et al. (2007) Does periodontal care improve glycemic control? The Department of Veterans Affairs Dental Diabetes Study. J Clin Periodontol 34: 46-52. [crossref]
- Iacopino AM (2001) Periodontitis and diabetes interrelationships: role of inflammation. Ann Periodontol 6: 125-137. [crossref]
- Southerland JH, Taylor GW, Offenbacher S (2005) Diabetes and periodontal infection: making the connection. Clin Diabetes 23: 171-178. [crossref]
- Taylor GW, Borgnakke WS (2008) Periodontal disease: associations with diabetes, glycemic control and complications. Oral Dis 14: 191-203. [crossref]
- PatinoMarın N, Loyola Rodrıguez JP, Medina Solis CE, Pontigo Loyola AP, et al. (2008) Caries, periodontal disease and tooth loss in patients with diabetes mellitus types 1 and 2. Acta Odontol Latinoam 21: 127-133. [crossref]
- Wang TT, Chen TH, Wang PE, Lai H, et al. (2009) A population-based study on the association between type 2 diabetes and periodontal disease in 12,123 middle-aged Taiwanese (KCIS No. 21). J Clin Periodontol 36: 372-379. [crossref]
- Nesse W, Linde A, Abbas F, Lucien Spijkervet FK, et al. (2009) Dose-response relationship between periodontal inflamed surface area and HbA1c in type 2 diabetics. J Clin Periodontol 36: 295-300. [crossref]
- Taylor GW (2001) Bidirectional interrelationships between diabetes and periodontal diseases: and epidemiologic perspective. Ann Periodontol 6: 99-112. [crossref]
- Grossi SG (2001) Treatment of periodontal disease and control of diabetes: an assessment of the evidence and need for future research. Ann Periodontol 6: 138-145. [crossref]
- Chandra A, Yadav OP, Narula S, Dutta A (2016) Epidemiology of periodontal diseases in Indian population since last decade. J Int Soc Prev Community Dent. 6: 91-96. [crossref]
- Shaju JP, Zade RM, Das M (2011) Prevalence of periodontitis in the Indian population: A literature review. J Indian Soc Periodontol 15: 29-34. [crossref]
- Sundar C, Ramalingam S, Mohan V, Pradeepa R, et al. (2018) Periodontal therapy as an adjunctive modality for HbA1c reduction in type-2 diabetic patients. J Educ Health Promot. 28;7: 152. [crossref]
- Singh S, Kumar V, Kumar S, Subbappa A (2008) The effect of periodontal therapy on the improvement of glycemic control in patients with type 2 diabetes mellitus: A randomized controlled clinical trial. Int J Diabetes Dev Ctries. 28: 38-44. [crossref]
- Kiran M, Arpak N, Unsal E, Erdoğan MF (2005) The effect of improved periodontal health on metabolic control in type 2 diabetes mellitus. J Clin Periodontol 32: 266-272. [crossref]
- World Health Organization (2006) Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. Geneva: World Health Organization,1-50.
- Stewart JE, Wager KA, Friedlander AH, Zadeh HH (2001) The effect of periodontal treatment on glycemic control in patients with type 2 diabetes mellitus. J Clin Periodontol 28: 306-310. [crossref]
- Cugini MA, Haffajee AD, Smith C, Kent RL Jr, et al. (2000) The effect of scaling and root planing on the clinical and microbiological parameters of periodontal diseases: 12-month results. J Clin Periodontol. 27: 30-36. [crossref]
- Nishimura F, Murayama Y (2001) Periodontal inflammation and insulin resistance-lessons from obesity. J Dent Res 80: 1690-1694.
- Moeintaghavi A, Arab HR, Bozorgnia Y, Kianoush K, et al. (2012) Non-surgical periodontal therapy affects metabolic control in diabetics: a randomized controlled clinical trial. Aust Dent J 57: 31-37. [crossref]
- Ryan ME (2008) Diagnostic and therapeutic strategies for the management of the diabetic patient. Compend Contin Educ Dent 29: 32-38, 40-44.
- Morita I, Inagaki K, Nakamura F, Noguchi T, et al. (2012) Relationship between periodontal status and levels of glycated hemoglobin. J Dent Res. 91: 161-166. [crossref]
- Sun WL, Chen LL, Zhang SZ, Ren YZ, et al. (2010) Changes of adiponectin and inflammatory cytokines after periodontal intervention in type 2 diabetes patients with periodontitis. Arch Oral Biol. 55: 970-974. [crossref]
- Venkataraman K, Kannan AT, Mohan V (2009) Challenges in diabetes management with particular reference to India. Int J Diabetes Dev Ctries 29: 103-109. [crossref]
- Petersen PE, Ogawa H (2005) Strengthening the prevention of periodontal disease: the WHO approach. J Periodontol. 76: 2187-2193. [crossref]
- Engebretson SP, Hyman LG, Michalowicz BS, Schoenfeld ER, et al. (2013) The effect of nonsurgical periodontal therapy on hemoglobin A1c levels in persons with type 2 diabetes and chronic periodontitis: a randomized clinical trial. JAMA 310: 2523-2532. [crossref]