DOI: 10.31038/CST.2019423
Abstract
PD-1 receptor as one of the programmed cell death protein 1 receptor was firstly designated in the early 1990s assumed its manifestation through out initiation of programmed cell death in a T-cell hybridoma. Subsequently its early detection, numerous groups have recognized that arrangement of PD-1 through its ligand, programmed death ligand 1 (PD-L1), negatively regulates T-cell-mediated immune responses. Early preclinical indication proposed that stimulation of PD-1/PD-L1 signaling might work as a mechanism for tumors to escape “an antigen-specific T-cell immunologic response”. Accordingly, the Atezolizumab is one of a novel immunotherapy medication called “checkpoint inhibitors”. It functioning through interfering with the tumor’s ability to deactivate cancer combat immune cells called (T-cells). It objects a “protein hypothesis was developed that PD-1/PD-L1 blockade may be an effective cancer immunotherapy.
For instance, several PD-1/PD-L1 inhibitors stays to develop, predictive biomarkers, mechanisms of resistance, management interval and therapy up on disease progression and immune-related side effects, are main ideasessential of additional attention to enhance the anticancer effects of this group of immunotherapy.
Keywords
PD-1/ PD-L1 inhibitor, Immune checkpoint, Treatment beyond progression, Immune-related toxicity
Introduction
The main role of T cells is to differentiate healthy cells from diseased or malignant cells by the activation or deactivation of numerous receptors on the T-cell surface. As stated before, the malignant cells can escape recognition through “cell surface molecules” that interact with the receptors on T cells to, in essence, mimic the signals released by healthy cells. So, the immune system that rests inactive against malignant cells, allowing their un-regulated development and proliferation.
As these molecules and their associated receptors on T cells keep the immune system ‘‘in check,” by “inhibiting immune functioning”, they are mutually called “checkpoint proteins”. The Checkpoint inhibitors inhibit the effects of these checkpoint proteins.
Types of checkpoint Inhibitors
Three different groups that targets different checkpoint proteins (1) PD-L1: Programmed Death Ligand-1, (2) PD-1: Programmed Cell Death Protein-1 and (3) CTLA-4; Cytotoxic T-Lymphocyte Associated Protein 4, have been the chief target of investigation for the management of cancer patients with immunotherapy as new era.CTLA-4 and PD-1 are found on T cells. PD-L1 are on cancer cells (Figure- 1).
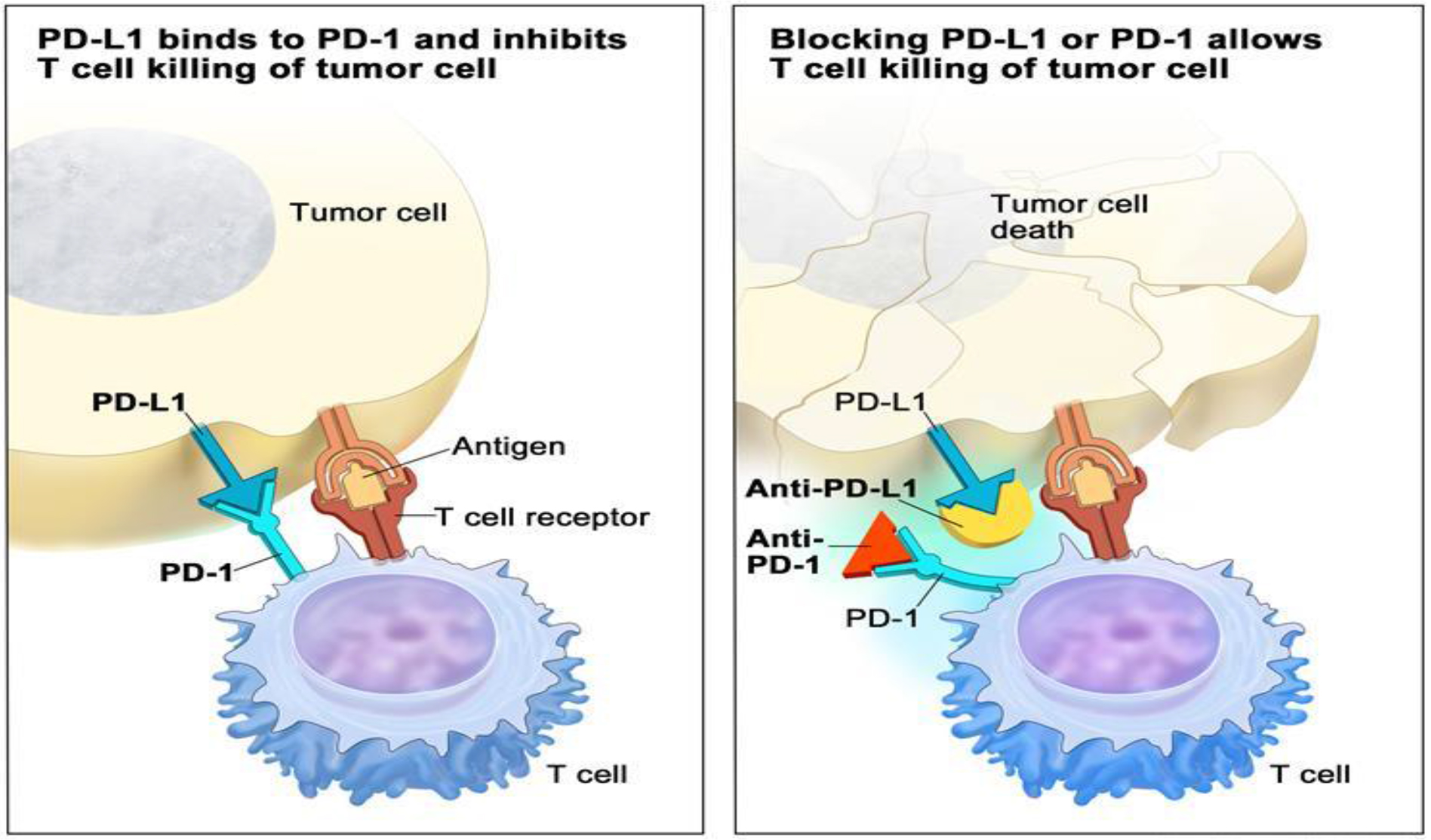
Figure 1. PD-1 and PD-L1 sites of action
Immune checkpoint inhibitor. Checkpoint proteins, such as PD-L1 on tumor cells and PD-1 on T cells, help keep immune responses in check. The binding of PD-L1 to PD-1 keeps T cells from killing tumor cells in the body (left panel). Blocking the binding of PD-L1 to PD-1 with an immune checkpoint inhibitor (anti-PD-L1 or anti-PD-1) allows the T cells to kill tumor cells (right panel).
https: //www.cancer.gov/publications/dictionaries/cancer-terms/def/immune-checkpoint-inhibitor.
PD-1 and its Immune-inhibitory Mechanism
The programmed cell death 1 (PD-1) receptor is expressed on activated T cells, B cells, macrophages, regulatory T cells (Tregs), and natural killer (NK) cells. Binding of PD-1 to its B7 family of ligands, programmed death ligand 1 (PDL1 or B7-H1) or PD-L2 (B7-DC) results in suppression of proliferation and immune response of T cells. Activation of PD-1/PD-L1 signaling serves as a principal mechanism by which tumors evade antigen-specific T-cell immunologic responses. Antibody blockade of PD-1 or PD-L1 reverses this process and enhances antitumor immune activity. TCR, T-cell receptor; MHC, major histocompatibility complex; APC, antigen-presenting cell.
PD-1 functions through regulation of late-phase immune responses. The management is as suggested by the activation-induced expression of PD-1. The control of immune reactions also takes place in the peripheral tissues. PD-1 contains single IgV-like domains as in its extracellular region. The region also includes ITIM and ITSM. PD-1 requires ligation with the physiological ligand to suppress T-cell activation (Figure- 1).
PD-1 Function
PD-1 and CTLA-4 can be applied at a diverse phase of immune reaction. They are also induced on the activated T-cells. PD-1 is shown on activated T-cells at a late effectors stage. In the condition of the chronic viral condition, there is a high persistence of PD-1 expression on CD8+ T-cells. Despite their distinct features, the CTLA-4 and PD-1 are both immune checkpoints.
They also regulate the immune responses at different stages. CTLA-4 can effectively block the activation of T-cells in the lymphoid organs. PD-1 functions effectively by inhibiting the effectors T-cells at a later stage of immune responses (Figure-2).
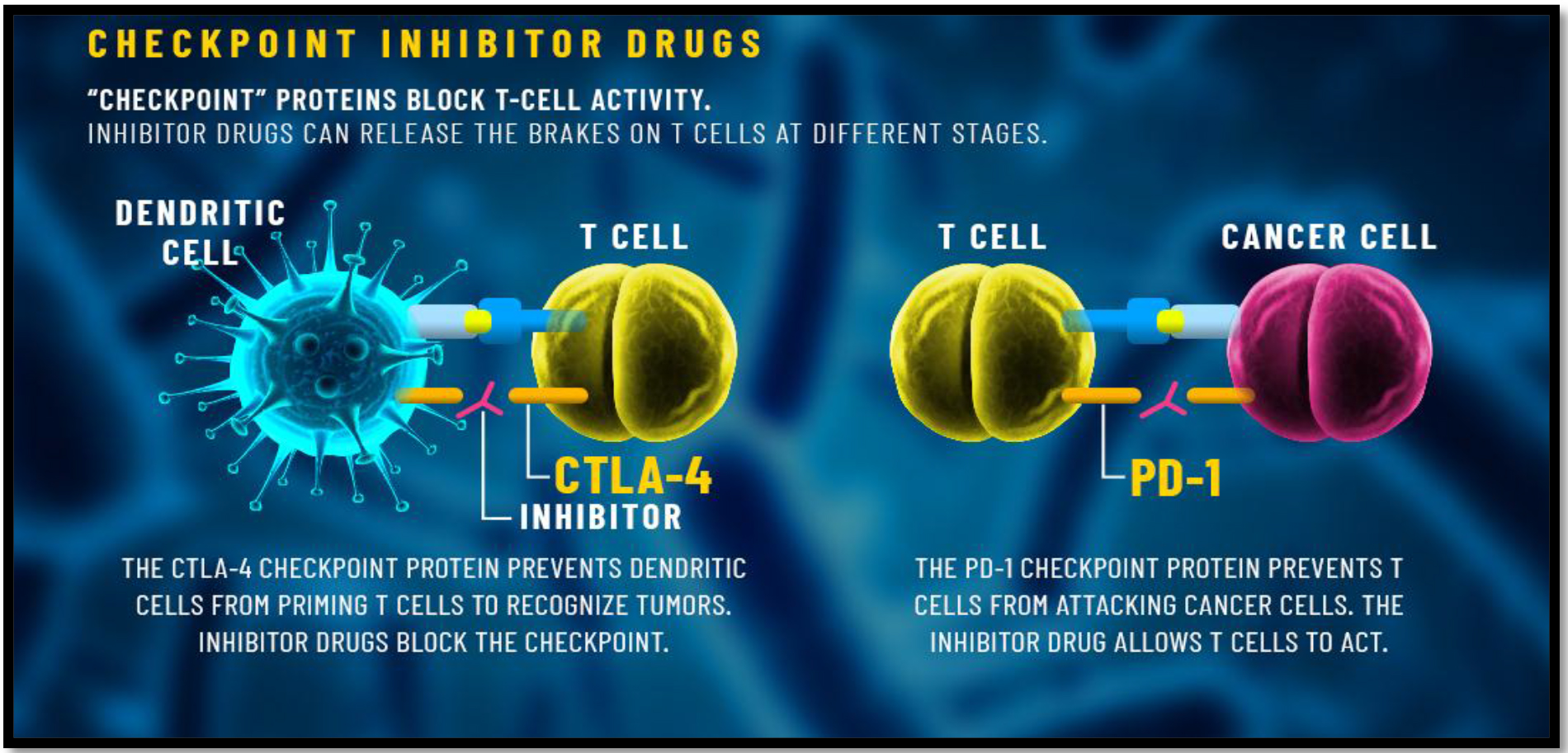
Figure 2. PD-1 and CTLA-4 sites of action
jim-Allison-who-first-used-CTLA4-blockade-for-cancer-treatment-and-Honjo-who-originally-discovered-PD-1-new-Nobel-Laureates-of-Medicine-this-year-Custom-Graphic
Control of Cancerous Immunity by PD-1
PD-1 is essential for dampening the immune-surveillance for tumours. Tumors can express the PD-L1 and thus escaping the immune surveillance. PD-L1 interacts with PD-1 on T-cells and therefore and thus negatively regulates the immune responses [5].
There is also a correlation between poor prognosis and high expression of PD-1 ligands on tumors. PD-1 blockade has previously successfully been used on metastatic tumors. Additionally, PD-1 has been identified to contain a higher therapeutic capacity than CTLA-4 blockade (Figure-3).
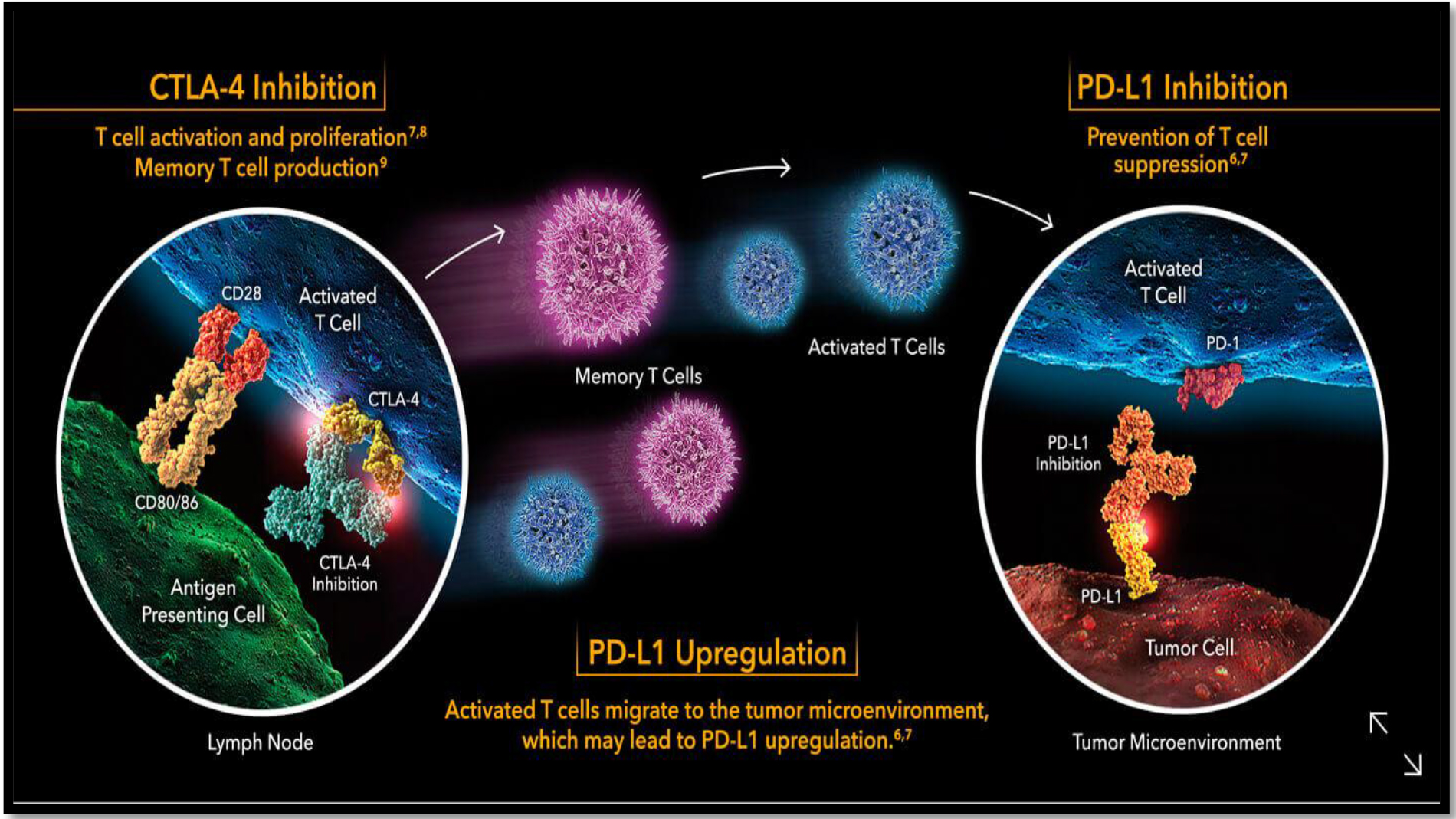
Figure 3. PD-1 Function and Action
jim-Allison-who-first-used-CTLA4-blockade-for-cancer-treatment-and-Honjo-who-originally-discovered-PD-1-new-Nobel-Laureates-of-Medicine-this-year-Custom-Graphic

Figure 4. PD-1 Inhibitors Side Effect
Role of PD-1 inhibitors in the treatment of cancer patients
1-Pembrolizumab (Keytruda) was developed by Merck and first approved by the Food and Drug Administration in 2014 for the treatment of melanoma. It was later approved for metastatic non-small cell lung cancer and head and neck squamous cell carcinoma. In 2017, it became the first immunotherapy drug approved for use based on the genetic mutations of the tumor rather than the site of the tumour.
Indications
- Indicated for the treatment of patients with unresectable or metastatic melanoma.
- Indicated for the adjuvant treatment of patients with melanoma with involvement of lymph node(s) following complete resection.
- In combination with pemetrexed and platinum chemotherapy, is indicated for the first-line treatment of patients with metastatic non-squamous non‒small cell lung cancer (NSCLC), with no EGFR or ALK genomic tumor aberrations.
- In combination with carboplatin and either paclitaxel or nabpaclitaxel, is indicated for the firstline treatment of patients with metastatic squamous NSCLC.
- As a single agent, is indicated for the first-line treatment of patients with metastatic NSCLC whose tumors have high PD-L1 expression [tumor proportion score (TPS) ≥ 50%] as determined by an FDA-approved test, with no EGFR or ALK genomic tumor aberrations.
- As a single agent, is indicated for the treatment of patients with metastatic NSCLC whose tumors express PD-L1 (TPS ≥1%) as determined by an FDA-approved test, with disease progression on or after platinum-containing chemotherapy. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations prior to receiving KEYTRUDA.
- Indicated for the treatment of patients with recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) with disease progression on or after platinum-containing chemotherapy. This indication is approved under accelerated approval based on tumor response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials.
- Indicated for the treatment of adult and pediatric patients with refractory classical Hodgkin lymphoma (cHL), or who have relapsed after 3 or more prior lines of therapy. This indication is approved under accelerated approval based on tumor response rate and durability of response.
- Indicated for the treatment of adult and pediatric patients with refractory primary mediastinal large B-cell lymphoma (PMBCL), or who have relapsed after 2 or more prior lines of therapy. This indication is approved under accelerated approval based on tumor response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
- Indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma (mUC) who are not eligible for cisplatin-containing chemotherapy and whose tumors express PD-L1 [combined positive score (CPS) ≥10], as determined by an FDA-approved test, or in patients who are not eligible for any platinum-containing chemotherapy regardless of PD-L1 status. This indication is approved under accelerated approval based on tumor response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
- Indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma (mUC) who have disease progression during or following platinum-containing chemotherapy or within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy.
- Indicated for the treatment of adult and pediatric patients with unresectable or metastatic microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR)solid tumours that have progressed following prior treatment and who have no satisfactory alternative treatment options, or colorectal cancer that has progressed following treatment with fluoropyrimidine, oxaliplatin, and irinotecan.
2-Nivolumab (Opdivo) was developed by Bristol-Myers Squibb and first approved by the FDA in 2014 for the treatment of melanoma.
Indications
- As a single agent is indicated for the treatment of patients with BRAF V600 mutation-positive un-resectable or metastatic melanoma. This indication is approved under accelerated approval based on progression-free survival. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials.
- As a single agent is indicated for the treatment of patients with BRAF V600 wild-type un-resectable or metastatic melanoma.
- In combination with YERVOY® (ipilimumab), is indicated for the treatment of patients with un-resectable or metastatic melanoma. This indication is approved under accelerated approval based on progression-free survival. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials.
- Is indicated for the treatment of patients with metastatic non-small cell lung cancer (NSCLC) with progression on or after platinum-based chemotherapy. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations prior to receiving OPDIVO.
- Indicated for the treatment of patients with advanced renal cell carcinoma (RCC) who have received prior anti-angiogenic therapy.
- Indicated for the treatment of adult patients with classical Hodgkin lymphoma (cHL) that has relapsed or progressed after autologous hematopoietic stem cell transplantation (HSCT) and brentuximabvedotin or after 3 or more lines of systemic therapy that includes autologous HSCT. This indication is approved under accelerated approval based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
- Indicated for the treatment of patients with recurrent or metastatic squamous cell carcinoma of the head and neck (SCCHN) with disease progression on or after platinum-based therapy.
- Indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma who have disease progression during or following platinum-containing chemotherapy or have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy. This indication is approved under accelerated approval based on tumor response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
- Indicated for the treatment of adult and pediatric (12 years and older) patients with microsatellite instability high (MSI-H) or mismatch repair deficient (dMMR) metastatic colorectal cancer (CRC) that has progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan. This indication is approved under accelerated approval based on overall response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
- Indicated for the treatment of patients with hepatocellular carcinoma (HCC) who have been previously treated with sorafenib. This indication is approved under accelerated approval based on tumor response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials.
- Indicated for the adjuvant treatment of patients with melanoma with involvement of lymph nodes or metastatic disease who have undergone complete resection.
3-Cemiplimab (Libtayo) is a human programmed death receptor-1 (PD-1) monoclonal antibody that binds to PD-1 and blocks its interaction with programmed death ligands 1 (PD-L1) and 2 (PD-L2). The drug is being investigated as a treatment for various cancers and in September 2018 received approval in the USA for the treatment of patients with metastatic cutaneous squamous cell carcinoma or locally advanced cutaneous squamous cell carcinoma who are not candidates for curative surgery or curative radiation. This article summarizes the milestones in the development of cemiplimab leading to this first global approval for the treatment of advanced cutaneous squamous cell carcinoma.
Side Effects of PD-1 Inhibitors
Side effects from treatment with checkpoint inhibitors typically appear within weeks or a few months of starting treatment but can persist or first appear after treatment has finished. Immune-related side effects (sometimes referred to as immune-related adverse effects or irAEs) arising from treatment with checkpoint inhibitors can affect any organ or tissue, but most commonly affect the skin, colon, lungs, liver and endocrine organs (such as the pituitary gland or thyroid gland) [7]. Most immune-related side effects are mild to moderate and reversible if detected early and addressed appropriately.
Management of side effects
There are other side effects to checkpoint inhibitors which occur infrequently, but of which you should be aware, as follows [7]:
- Neurological symptoms – according to an analysis of data from many clinical trials, these occur in approximately 4%–6% of people treated with CTLA-4 inhibitors or PD-1 inhibitors, or in up to 12% if treated with both types in combination, and manifests in a wide range of different ways (including muscle weakness, numbness and breathing difficulties); treatment for symptoms of Grade 2 or higher is based mainly on increasing strength oral or intravenous corticosteroids.
- Rheumatological symptoms – mild or moderate muscle or joint pain occurs in 2%–12% of people treated with checkpoint inhibitors, more commonly with PD-1 inhibitors; treatment is mainly with oral analgesics (mild-to-moderate symptoms), low-dose oral corticosteroids (moderate symptoms), or for severe symptoms, consultation with a specialist and high-dose corticosteroids or intravenous immunosuppressive drugs may be necessary. Treatment with checkpoint inhibitors may need to be interrupted or stopped, depending on symptom severity.
- Kidney symptoms – fewer than 1% of people treated with CTLA-4 inhibitors or PD-1 inhibitors experience kidney problems (although approximately 5% do so if treated with the two types of checkpoint inhibitors in combination); significant impairment of kidney function is treated with intravenous corticosteroids and specialist intervention, and may require checkpoint inhibitor treatment to be interrupted or stopped.
- Cardiac symptoms – seen in less than 1% of people treated with CTLA-4 inhibitors or PD-1 inhibitors and includes a wide range of different types; these require early referral to a cardiologist and treatment with high-dose corticosteroids or other immunosuppressive drugs.
Conclusion
In the immunotherapy era, PD-1 inhibitors achieved strong response for many cancer patients either as an adjuvant or palliative treatment. The side effects appear as mild to moderate and can be managed as discovered early.
References
- Khanna P, Blais N, Gaudreau P, Corrales-Rodriguez L (2017) Immunotherapy Comes of Age in Lung Cancer. Clinical Lung Cancer. 2017; 18(1): 13–22.
- Iwai Y, Hamanishi J, Chamoto K, HonjoT (2017) Cancer immunotherapies targeting the PD-1 signaling pathway. Journal of Biomedical Science. 2017; 24(1).
- Zhang R, Li P, Li Q, Qiao Y and Xu T et al (2018) Radiotherapy improves the survival of patients with stage IV NSCLC: A propensity score matched analysis of the SEER database. Cancer Medicine. 2018; 7(10): 5015–5026.
- Almutairi A, Alsaid N, Martin J, Babiker H and McBride A et al (2018) Comparative efficacy and safety of immunotherapies targeting PD-1/PD-L1 pathway for previously treated advanced non-small cell lung cancer: Bayesian network meta-analysis. Journal of Clinical Oncology. 2018; 36(15_suppl): e21012-e21012.
- Ferris R. PD-1 targeting in cancer immunotherapy (2012) Cancer.; 119(23): E1-E3.
- TogashiY (2017). ISY9-2Translational research for predictive biomarkers and novel cancer immunotherapies beyond PD-1/PD-L1 blockade therapies. Annals of Oncology. 2017; 28 (suppl_9).
- Haanen JBAG, Carbonnel F, Robert C, et al (2017) Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017; 28(suppl_4): iv119-iv142.