Abstract
Background and aims: The evaluation of the insecticidal activity of plants constitutes a novel approach for the development of new natural and bioavailable products for the control of vectors. The objective of this work was to evaluate the ovicidal activity and the repelllency to oviposition of Aedes aegypti and Aedes albopictus at different breeding sites of six essential oils; Bursera graveolens (Kunth) Triana & Planch, Citrus aurantium L., Eucalyptus globulus Labill, Melaleuca quinquenervia (Cav.) ST Blake, Ocimum basilicum L., Piper aduncum subsp ossanum (C. DC.) Saralegui.
Methods: Two strain of Ae. aegypti (susceptible and one from the field), one strain of Ae. albopictus collected in one municipality of Havana province and six essential oil were used during the study. The ovicidal activity and the repellency for the selection of the oviposition sites of the oils were determined following the methodology described by the literature.
Results: There was low ovicidal activity of all the evaluated oils, on Ae. aegypti and Ae. albopictus. All the essential oils generated repellency to the oviposition in both species, being C. aurantium and P. aduncum subsp ossanum those that showed a total repellence.
Conclusions: With knowledge of the behavior of oviposition of mosquitoes and the mode of action of essential oils from Cuban flora, formulations could be developed to contribute to the ovicidal action and / or to discourage the oviposition of these insects.
Keywords
Mosquitoes, Piper, Citrus, Bursera, Eucalyptus, Ocimum, Melaleuca, chemoreception
Introduction
In the last decade, arboviruses such as, dengue, chikungunya and zika show a tendency to increase their incidence and spread to new geographic areas [1], including the reemergence of yellow fever in several countries of the Americas [2]. One of the causes of the spread of these viruses is attributed to the geographic expansion of Aedes aegypti (Linnaeus, 1762) which is an effective vector of various arboviruses. Its major epidemiological importance is linked to its role as a transmitter of dengue and yellow fever, in addition to Zika [1]. On the other hand Aedes albopictus (Skuse, 1895) is another mosquito of great epidemiological importance. This species has been detected naturally infected with DENV-1 and DENV-2 in Colombia [3–4] and in Ceará, Brazil by DENV-2 and DENV-3 [5], so its participation in the dynamics of transmission of dengue to humans is not it can be discarded. It was also linked to chikungunya outbreaks in Reunion Island, French Republic [6] in 2003 and with outbreaks in 2007 in northern Italy [7] and Spain [8] as a result of the invasion and expansion of this species since the beginning of the 2000s in these countries.
Unfortunately, due to the lack of effective vaccines (except for yellow fever) that allow us to protect the human population, the application of insecticides is the most widely used strategy to reduce the incidence of the rest of arboviruses diseases [9–10].
Despite the availability of chemical formulations and equipment [11], in many geographical areas, the chain of transmission cannot be stopped. Among the elements that propitiate it are; operational factors, the lack of involvement of the community, the resistance of the vectors to available insecticides, among others [12]. The World Health Organization (WHO) motivates the search and implementation of new control alternatives [13], so that the development of new products natural and bioavailable for our country constitutes a novel approach with possible practical applications in Cuba.
Some authors study the insecticidal activity of plants, but there are few studies on the ovicidal activity of plant extracts and essential oils as a form of vector control [14], without discarding the repellent effect that a natural product could cause in the selection of oviposition sites [15].
Due to this, the objective in this work was to evaluate the ovicidal activity of six essential oils on eggs of Ae. aegypti and Ae. albopictus, in addition to the repellency in the selection of oviposition sites by both mosquito species.
Materials and Methods
Biological material used in the research
For the study eggs from the:
- Rockefeller strain (Ae. aegypti): reference strain susceptible to insecticides, supplied by the Center for Disease Control and Prevention (CDC), San Juan, Puerto Rico, 1996.
- Marianao population 2013 (Ae. aegypti): collected in larva and pupa stages, in low tank containing temefos, Marianao municipality, Finlay health area, Havana, Cuba, in 2013 during an intensive phase of vector control.
- Fraga 2012 population (Ae. albopictus): larvae and pupa collected in a surveillance device (larvitrampa) in Juan de Dios Fraga neighborhood, Pulido Humaran health area, La Lisa, Havana, Cuba, 2012.
The plants used in the study were the following:
- Bursera graveolens (Kunth) Triana & Planch. (Burseraceae) Common name: Sasafrás. It was collected in La Lisa, Havana, 2014. The herborization was taken as reference [16] because it is a sample, from the same source.
- Citrus aurantium L. (Rutaceae), common name: sweet orange. The oil was obtained industrially in the Combinado Citrícola Victoria de Girón, Jagüey Grande, Matanzas (Lot 13700, year 2016).
- Eucalyptus globulus Labill (Myrtacaeae) Common name: eucalyptus. It was collected, between the months of November and December of the year 2014 in the IFAL. A representative specimen was herborized and deposited in the Institute of Ecology and Systematics HB 88667.
- Melaleuca quinquenervia (Cav.) S. T. Blake (Myrtaceae) common name: melaleuca. It was collected in the Laguna del Tesoro, Ciénaga de Zapata, 2011. A representative specimen was herborized and deposited in the Institute of Ecology and Systematics with the identification HB 42678.
- Ocimum basilicum L. (Lamiacaeae), common name: basil. It was collected, between the months of December of the year 2014 in the IFAL. A representative specimen was herborized and deposited in the National Botanical Garden HFC-087057.
- Piper aduncum subsp ossanum (C. DC.) Saralegui (Piperaceae) common name: platanillo. It was collected, between the months of September of the year 2013 in the Artemisa province. A representative specimen was herborized and deposited in the National Botanical Garden HFC-87641.
The essential oils of B. graveolens, E. globulus, O. basilicum and P. aduncum subsp ossanum were extracted by hydrodistillation and M. quinquenervia was extracted by steam trawling from the aerial parts. Citrus aurantium, was obtained by expression, from the pericarp of the fruit. The methods used to obtain the oils were governed by the ISO 65–71: 84 standard of the Ministery of Public Health in Cuba [17]. The essential oils were kept at 4°C until the preparation of the solutions for the corresponding bioassays.
The chemical composition of the essential oils was determined by gas chromatography coupled to mass spectrometry (GC-MS), in a gas chromatograph of the series Agilent 6890 with an injector of the type “split splitless” (split ratio 20: 1) coupled with a mass spectrometer of the Agilent 05973 series (both from Agilent Technologies, Palo Alto, CA, USA). The identification of the compounds was carried out through the combined use of the automated databases NBS-NISTASCI and Wiley 275 and the Atlas Registry of Mass Spectra Dat
Determination of the ovicidal activity of essential oil solutions against Ae. aegypti and Ae. albopictus.
The ovicidal activity was determined following the methodology of Prajapathi et al., [18]. Filter paper strips were used that contained an average of 300 oviposited eggs a week previously observed under the stereoscope to confirm the absence of collapse and the presence in appearance of the embryo. The filter paper strips with eggs were exposed to the lethal concentration of the oils that caused 90% mortality of the previously treated individuals from each population (CL90) (Table 1). The eggs were immersed in containers containing 1 mL of each oil solution (those prepared in ethanol) in 99 mL of dechlorinated water for 24 hours. A control was used, which consisted of exposing eggs to 1 mL of ethanol in 99 mL of dechlorinated water. 1 control and three replications were used for oil. Later the strips with eggs were extracted from the medium, placed to dry in a tray at 25oC and the total number of eggs hatched under a stereoscope was counted.
Table 1. Lethal concentrations values CL90 (mg / L) and reliability limits obtained in previous studies with the Rockefeller strain and the populations Marianao 2013 (Ae. aegypti) and Fraga 2012 (Ae. albopictus).
|
Essentials Oils |
Rockefeller |
Marianao 2013 |
Fraga 2012 |
|
Bursera graveolens |
27,4 |
64,3 |
58,5 |
|
Ocimun basilicum |
87,6 |
93,3 |
27,0 |
|
Melaleuca quinquenervia |
59,2 |
140 |
89,4 |
|
Eucalyptus globulus |
81,8 |
15,5 |
128,7 |
|
Piper aduncum subsp. ossanum |
63,5 |
64,8 |
82,3 |
|
Citrus aurantium |
19,5 |
22,3 |
52,5 |
For the analysis of the normality of the data the Shapiro Wilk test was used and for the analysis of the data an ANOVA (p <0.05). The Statistica version 7 program was used.
Determination of oviposition repellency in populations of Ae. aegypti and Ae. albopictus before essential oil solutions.
To determine the oviposition repellency before oil solutions, 3 replicas were used for each population and for oil. In each cage, 25 females and 25 males were placed and three containers containing:
- 500 mL dechlorinated water,
- 500 mL dechlorinated water with 20 second stage larvae to simulate hatchery
- Another container containing 1 mL of oil solution in 499 mL of dechlorinated water.
They were given blood feed for 3 hours 2 times a week. A strip of paper was placed on the containers to collect the egg. The paper filter strips with eggs were extracted and placed on a wet surface in trays for 24 hours to allow the proper development of embryogenesis and then put to dry at 25 oC. Subsequently, they were placed in dechlorinated water with 0.1 g of fishmeal to promote hatching. At 24 hours, under a stereoscope, the total number of eggs was recorded as fecundity and the fraction of eggs hatched as fertility. The “Student’s T” test was applied to compare the mean of the eggs laid and hatched for each species (p <0.05), using the statistical package Statistica 7.0
Results
Ovicidal activity of essential oils on Ae. aegypti and Ae. albopictus
When submitting eggs from the populations of Ae. aegypti and Ae. albopictus at CL90, a significant difference was found in the hatching percentage of the controls with respect to those exposed (Figure 1). Although hatching was greater than 50%, no larvae survived the exposed concentrations.
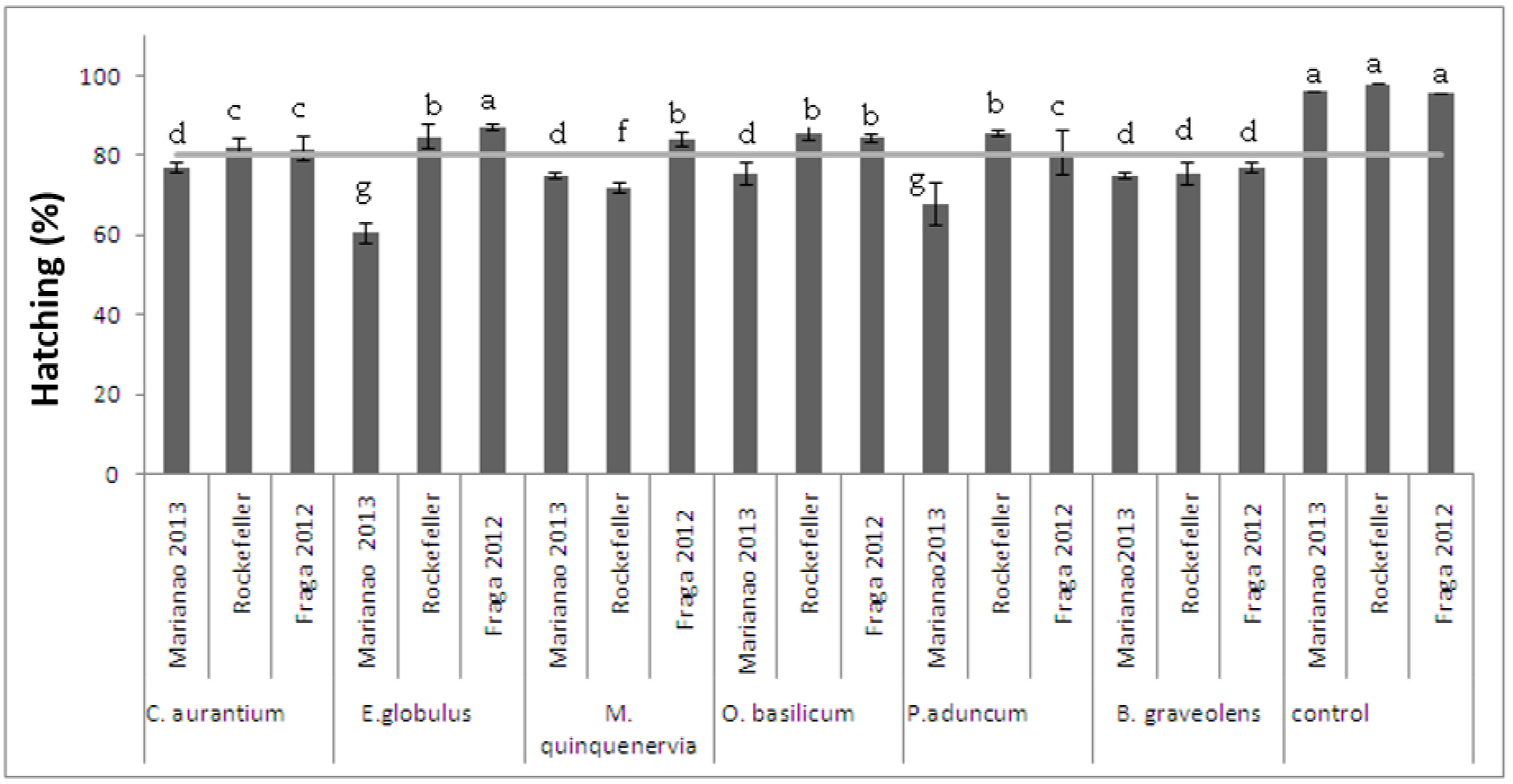
Figure 1. Ovicide activity of essential oils on eggs of the Rockefeller strain and the Marianao 2013 (Ae. aegypti) and Fraga 2012 (Ae. albopictus) populations F (10,30) = 4,27 p = 0,00094. Stocks with a common letter are not significantly different (p> 0.05)
Oviposition repellency of Ae. aegypti and Ae. albopictus before solutions of essential oils.
In the study carried out, significant differences were found regarding oviposition in the solutions of the different essential oils (F =, 06625 p = 1.0000). The populations used preferred egg laying in dechlorinated water and dechlorinated water containing larvae, rather than placing the eggs in solutions of the essential oils (Figure 2). Citrus aurantium and P. aduncum subsp.ossanum provoked total oviposition repellency on the part of the females of both Aedes species. Oviposition in the solutions of the rest of the oils occurred with an increasing trend in B. graveolens, O. basilicum, E. globulus and M. quinquenervia but at very low percentages, mostly less than 8%.
The chromatographic analysis of the samples showed that C. aurantium oil, used in this study, showed a relative abundance of 97.5% for limonene and 1.5% myrcene. For B. graveolens, the compounds with the highest relative abundance (> 1%), limonene (21.8%) and β-elemene (12.5%) were identified. For E. globulus, eucalyptol and p-cymene accounted for 63.1% of the oil, followed by γ terpinene (16.7%), 4-terpineol (6.6%) and thymol (1.4%). In the essence of O. basilicum evaluated, estragole (50.8%), linalool (30.3%) and 1,8-cineol (6.2%) were identified as major components. For M. quinquenervia we identified 1.8 cineol (28.8%),viridiflorol (25.3%), together with α-pinene (8.9%), β-pinene (2.2%), and limonene (13.6%). The oil of P. auncum subsp ossanum highlighted a relative abundance of linalool (32.6%), estragole (43.4%), 1,8 cineol (5.9%)
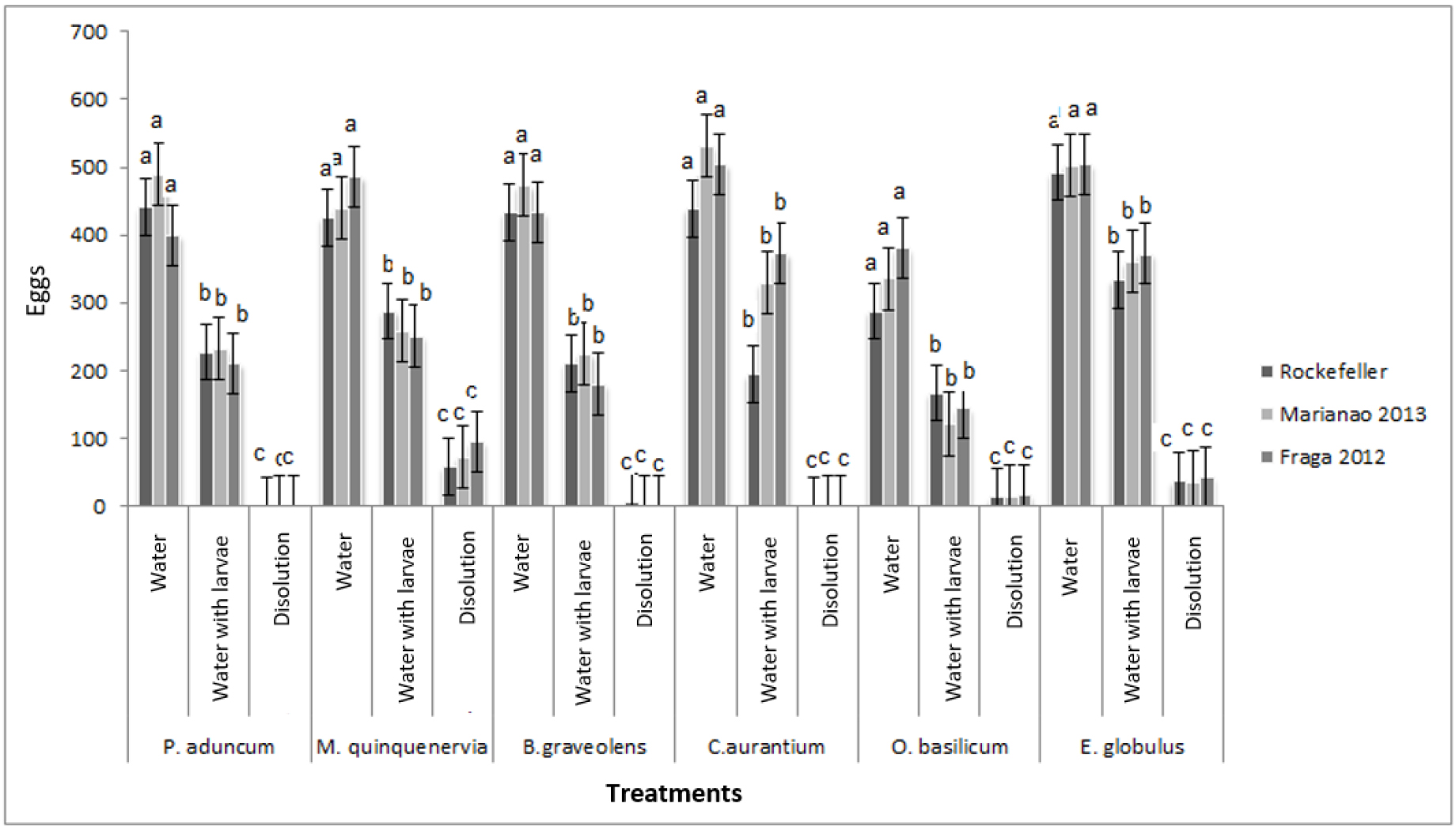
Figure 2. Oviposition repellency against different oil solutions on eggs of the Rockefeller strain and the Marianao 2013 (Ae. aegypti) and Fraga 2012 (Ae. albopictus) populations used in the study. F (21,184) = 0.66 p = 1.0000. Stocks with a common letter are not significantly different (p> 0.05)
Discussion
Ovicide activity of essential oil solutions on Ae. aegypti and Ae. albopictus
The high percentage of hatching at the dose used showed low ovicidal action of the oils evaluated. With P. aduncum, B. graveolens and E. globulus, no studies were found that allowed us to compare our results. Studies carried out with O. basilicum [20,21] show that this oil inhibited the hatching of Ae. aegypti and Cx. quinquefasciatus to different in 45 and 79% respectively.
Some authors suggest that the mechanism of general action that essential oils possess is given by their lipophilic nature [20]. The components present in the oils interact with cellular lipid membranes, destabilizing their integrity and that of other structures rich in lipopolysaccharide molecules. It has also been proposed the spontaneous formation of molecular complexes that facilitate an increase in cellular permeability favoring the circulation of ions and macromolecules and causing death due to functional failure in the organism [22,23].
The mosquito eggs have an outer membrane called chorion that confers protection to the embryo, which allows, among other functions, the exchange of gases and water with the outside through the aeropils [24,25]. It has been described that the ovicidal compounds can cross this barrier, block the hatching of the egg or interrupt the development of the embryo, and thus the survival of the larva inside the egg [26]. Most of the studies with essential oils evaluating the ovicidal activity, pose the bioactivity found, but not the mechanism by which this effect is provoked [27,28]. However, what was previously raised by other authors about the lipoficity of oils and the interaction with lipid membranes [21] could be related to the mechanism of action.
Studies carried out by Jarial et al. (2001) showed that the non-detachment of the exocorion in the eggs after being exposed to extracts of Allium sativum caused the non-hatching of the embryos [29]. Some authors [30,31] suggest that once the eggs harden, they are waterproofed, so the ovicidal action becomes more difficult as the time of oviposition increases, achieving an inversely proportional relationship between concentration and hatching in young eggs. De Lima Santos et al., (2013) [32] propose the interaction between the lectin of M. oleifera with the chitin present in the oocytes and the chorion of the eggs as an ovicidal mode of action, achieving the dissolution of the embryo in eggs of different times of life and subjected to the ovicide solution for 72 hours.
The doses used in this work, caused a high percentage of hatching of the exposed eggs and caused the subsequent death of the newly hatched larvae. We can infer that the essential oil solutions studied were able to cross the barriers of the chorion, causing the larvae to emerge as a survival mechanism. However, the same condition that led to the emergence was the same medium for which they emerged, which caused the death of first-stage individuals as a secondary effect. Although there are no studies of ovicidal activity with isolated compounds, several authors report activity on mosquito larvae of compounds present in the oils studied. Metabolites such as limonene and 1,8 cineol [34], estragole and linalool [35,36], b-elemeno [37] and p-cimeno [36] manifest insecticidal activity on immature stages of mosquitoes. In depth studies should be done later with lower doses and their effect on embryos.
Oviposition repellency of Ae. aegypti and Ae. albopictus before essential oil solutions
In the case of adult mosquitoes, the capacity for chemo-perception is well established through their tarsals and maxillary palps, which allows them to capture synthetic and natural chemicals at oviposition sites [38–40]. In the present investigation, the oil of C. aurantium, totally inhibited the oviposition by females of Ae. aegypti, however, like the rest of the oils evaluated, did not act as an ovicide. Kassir et al., (1989) [41] suggested that water treated with limonene (the majority compound present in this essence) was unfavorable for the oviposition of Cx. quinquefasciatus females. Similar results were found for Ae. Aegypt I [42].
The oil of P. aduncum subsp. ossanum showed similar results as C. aurantium. The essential oil obtained from P. nigrum, showed a moderate degree of dissociation at oviposition (82%) and P. marginatum, although it showed effectiveness as a larvicide, it did not interfere significantly in the oviposition of Ae. Aegypti [43–44].
Other studies have shown a high dissociation of oviposition with Melaleuca cajeputi (87.9%) on Ae. Aegypti [43]. Warikoo et al., (2011) found a directly proportional relationship between the evaluated concentration of O. basilicum and the effective repellency of Ae. aegypti to oviposit19. Similarly, it was found in studies with the essential oils of Lippia alba ((Mill.) NEBr. Ex Britton & P. Wilson), Corymbia citriodora ((Hook.) KD Hill & L.A.S Johnson) and Cananga odorata ((Lam.) Hook.f. & Thomson) that gravid females of Ae.aegypti showed some repellency to deposit their eggs in the treatments when compared with the controls15.
Studies carried out with the oil of M. quinquenervia produced a repellent and dissociative effect of the minced [45], and yet in our results offered partial repellency in the oviposition vessel. This result coincides with studies with the hexanic extract of leaves of Moringa oleifera Lamarck where they reported repellency to the bite of An. stephensi [46], while other studies [32] found a moderate dissociation to the oviposition of the lectin isolated from this plant against Ae. aegypti.
The variations in the repellent responses can be modulated mainly by the chemical composition of the oils and their major components, in addition to the response to the chemical signals received by the sensory organs that can vary according to the species of mosquito. There are families of plants that show high repellent activity [47] given by compounds such as α-pinene, limonene, citronellol, citronellal, camphor and thymol [48] where the presence of major and minor active compounds can generate additive or synergistic effects within the oils [49–50].
Conclusion
The doses used in this work proved to be effective in the protection of containers that these vectors use for their breeding, since it discourages females from continuing oviposition and in the event that an oviposition occurs in unprotected containers, at the time of application It will be able to favor the hatching of the eggs and eliminate the newly emerged larvae. With knowledge of the behavior of oviposition of mosquitoes and the mode of action of essential oils from Cuban flora, formulations could be developed to contribute to the ovicidal action and / or to discourage the oviposition of these insects.
References
- PAHO (2017) Pan American Health Organization / World Health Organization. Zika – Epidemiological Report Cuba. Washington, D.C.: PAHO/WHO; 2017.
- OPS Organización Panamericana de la Salud / Organización Mundial de la Salud. Actualización Epidemiológica: Fiebre amarilla,7 de diciembre de 2018, Washington D.C. OPS/OMS. 2018.
- Méndez F, Barreto M, Árias JF, Rengifo G, Muñoz J, et al. (2006) Human and mosquito infections by dengue viruses during and after epidemics in a dengue-endemic region of Colombia. Am JTrop Med Hyg 74: 678–683.
- Gómez-Palacio A, Suaza-Vasco J, Castaño S (2017) Infección de Aedes albopictus (Skuse, 1894) con el genotipo asiático-americano del virus del dengue serotipo 2 en Medellín y su posible papel como vector del dengue en Colombia. Biomédica 37: 135–142.
- Martins VE, Alencar CH, Kamimura MT, de Carvalho Araújo FM, De Simone SG, et al. (2012) Occurrence of natural vertical transmission of dengue-2 and dengue-3 viruses in Aedes aegypti and Aedes albopictus in Fortaleza, Ceará, Brazil. PLoS One 7: 41386.
- Pialoux G, Gaüzère BA, Jauréguiberry S, Strobel M (2007) Chikungunya, an epidemic arbovirosis. Lancet Infect Dis 7: 319–327. [crossref]
- Seyler T, Rizzo C, Finarelli AC, Po C, Alessio P, et al. (2008) Autochthonous chikungunya virus transmission may have occurred in Bologna, Italy, during the summer 2007 outbreak. Euro Surveill 13 (Suppl 3).
- Sánchez-Seco MP, Negredo AI, Puente S, Pinazo MJ, Shuffenecker I, et al. (2009) Diagnóstico microbiológico del virus chikungunya importado en España (2006–2007): detección de casos en viajeros. Enferm Infecc Microbiol Clin 27: 457–461.
- WHO Global strategic framework for integrated vector management. WHO, Geneva, Switzerland http://whqlibdoc.who.int/hq/2004/WHO_CDS_CPE _PVC_ 2004_10.pdf
- WHO. Global insecticide use for vector-borne disease control 5th ed. WHO/HTM /NTD /VEM/WHOPES/2011.6.
- WHO Equipment for vector control specification guidelines, second edition WHO/CDS/NTD/WHOPES/2018.02.
- George L, Lenhart A, Toledo J, Lazaro A, et al. (2015) Community-effectiveness of temephos for dengue vector control: a systematic literature review. PLoS Negl Trop Dis 9: e0004006.
- WHO (2017) Integrating neglected tropical diseases into global health and development: fourth WHO report on neglected tropical diseases.2017 http://www.who.int/neglected_diseases/en WHO/HTM/NTD/2017.01
- Gnankine O, Bassole IHN (2017) Essential oils as an alternative to pyrethroids’resistance against anopheles species complex giles (Diptera: Culicidae). Molecules 22: 1321.
- Castillo RM, Stashenko E, Duque JE (2017) Insecticidal and repellent activity of several plant-derived essential oils against Aedes aegypti. J Amer Mosq Control Assoc 33: 25–35.
- Monzote L, Hill GM, Cuellar A, Scull R, Setzerb WN (2012) Chemical Composition and Anti-proliferative Properties of Bursera graveolens. Essential Oil Natural Product Communications 7.
- ISO International Standarization Organization. ISO 65–71. Spices, condiments and herbs. Determination of volatile oil content. 1984. (Norma ISO).
- Prajapathi V, Tripathi AK, Aggarwall KK, Kanuja SP (2005) Insecticidal repellent and oviposition-deterrente activity of selected essential oils against An. stephensi, Ae. aegypti and Cx. quinquefasciatus. Bioresour Technol 96: 1749–57.
- Warikoo R, Wahab N, Kumar S (2011) Oviposition-altering and ovicidal potentials of five essential oils against female adults of the dengue vector, Aedes aegypti L Parasitol Res 109: 1125–1131. [crossref]
- Valarmathy D, Govindaraj M, Elumalai K (2011) Studies on Ovicidal activity of plant essential oil frommulation against the eggs of important vector mosquitoes, Anopheles stephensi (Liston), Culex quinquefasciatus (Say) and Aedes aegypti (L.) at laboratory condition. Int J Curren Res 6: 378–381.
- Lomonaco, D, Santiago, GMP, Ferreira, YS, Arriaga, AMC, et al. (2009) Study of technical CNSL and its main components as newgreen larvicides. Green Chem 11: 31–33.
- Toloza AC, Zygadlo J, Cueto GM (2006) Fumigant and repellent properties of essential oils and component compounds against permethrin resistant Pediculus humanus capitis (Anoplura: Pediculidae) from Argentina. J Med Entomol 43: 889–895.
- Isman M, Miresmailli S, Machial C (2011) Commercial opportunities for pesticides based on plant essential oils in agriculture, industry and consumer products. Phytochem Rev 10: 197–204.
- Russell BM, Kay BH, Shipton W (2001) Survival of Aedes aegypti (Diptera: Culicidae) eggs in surface and subterranean breeding sites during the Northern Queensland dry season. J Med Entomol 38: 441–445.
- Moreira MF, Dos Santos AS, Marotta HR, Mansur JF, Ramos IB, et al. (2007) A chitin-like component in Aedes aegypti eggshells, eggs and ovaries. Insect Biochem Mol Biol 37: 1249–1261. [crossref]
- Govindarajan M, Mathivanan T, Elumalai K, Krishnappa K, Anandan A (2011) Ovicidal and repellent activities of botanical extracts against Culex quinquefasciatus, Aedes aegypti and Anopheles stephensi (Diptera: Culicidae). Asian Pacific J Trop Biom 1: 43–48.
- Krishnappa K, Elumalai K (2013) Mosquitocidal properties of Basella rubra and Cleome viscosa against Aedes aegypti (Linn.) (Diptera:Culicidae) European Review for Medical and Pharmacological Sciences 17: 1273–1277.
- Reegan AP, Gandhi MR, Paulra MG, Ignacimuthu S (2015) Ovicidal and Oviposition deterrent activities of medicinal plant extracts against Aedes aegypti L. and Culex quinquefasciatus Say mosquitoes (Diptera: Culicidae) Osong Public Health Res Perspect 6: 64–69
- Jarial MS (2001) Toxic Effect of Garlic Extracts on the Eggs of Aedes aegypti (Diptera: Culicidae): A Scanning Electron Microscopic Study. J Med. Entomol 38: 446–450.
- Govindarajan M, Jebanesan A, Pushpanathan T (2008) Larvicidal and ovicidal activity of Cassia fistula Linn. leaf extract against filarial and malarial vector mosquitoes. Parasitol Res 102: 289–292.
- Kuppusamy C, Murugan K (2008) Mosquitocidal effect of Euphorbia heterophylla Linn. against the bancroftian filariasis vector, Culex quinquefasciatus Say. (Diptera: Culicidae). Int J Intg Boil 4: 34–39.
- De Lima Santos ND, Santana K, Napoleao TH, Novais Santos G, Breitenbach LC, et al. (2013) Oviposition stimulant and ovicidal activities of Moringa oleifera lectin on Aedes aegypti. Plos One 7: 44840.
- Santos G, Dutra K, Lira C, Lima B, Napoleão Th, et al. (2014) Effects of Croton rhamnifolioides essential oil on Aedes aegypti oviposition, larval toxicity and trypsin activity. Molecules 19: 16573–16587.
- Zahran HEDM, Abd El Galeil SAM (2011) Insecticidal and developmental inhibitory properties of monoterpenes on Cx. pipiens L. (Diptera: Culicidae). J Asia Pacific Entomol 14: 46–51.
- Govindarajan M (2010) Chemical composition and larvicidal activity of leaf essential oil from Clausena anisata (Willd.) Hook. f. ex Benth (Rutaceae) against three mosquito species. Asian Pacific Journal of Tropical Medicine 874–877.
- Giatropoulos A, Kimbaris A, Michaelakis A, Papachristos D, Polissiou MG, et al. (2018) Chemical composition and assessment of larvicidal and repellent capacity of 14 Lamiaceae essential oils against Aedes albopictus. Parasitol Res 117: 1953–1964.
- Govindarajan M, Rajeswary M, Arivoli S (2016) Larvicidal and repellent potential of Zingiber nimmonii (J. Graham) Dalzell (Zingiberaceae) essential oil: An eco-friendly tool against malaria, dengue, and lymphatic filariasis mosquito vectors? Parasitol Res 115: 1807–1816.
- Davis EE, Bowen MF (1994) Sensory physiological basis for attraction in mosquitoes. J Am Mosq Control Assoc 10: 316–325.
- Olagbemiro TO, Birkett MA, Mordue AJ, Pickett JA (1999) Production of (5-R, 6-S)-6-acetoxy-5-hexadecanolide, the mosquito oviposition pheromone, from the seed oil of the summer cypress plant, Kochia scoparia (Chenopodiaceae). JAgric Food Chem 47: 3411–3415.
- Geetha I, Pailey P, Padmanaban V, Balaraman K (2003) Oviposition,response of the mosquito, Culex quinquefasciatus to the secondary metabolite(s) of the fungus, Trichoderma viridae. Mem Inst Oswaldo Cruz 98: 223–26.
- Kassir JT, Mohsen ZH, Mehdi NS (1989) Toxic effects of limonene against Culex quinquefasciatus Say larvae and its interference with oviposition. Anz Schidlingskde, Pflanzenschutz, Umweltschutz 62: 19–21.
- Araujo FDO, Ribeiro-Paes J, Telles de Deus J, Holanda Cavalcanti SC, de Souza Nunes R, et al. (2016) Larvicidal activity of Syzygium aromaticum (L.) Merr and Citrus sinensis (L.) Osbeck essential oils and their antagonistic effects with temephos in resistant populations of Aedes aegypti. Mem Inst Oswaldo Cruz 111: 443–449.
- Tawatsin AA, U Thavara, P Wongsinkongman, J Bansidhi, T Boonruad, et al. (2006) Repellency of essential oils extracted from plants in Thailand against four mosquito vectors (Diptera: Culicidae) and oviposition deterrent effects against Aedes aegypti (Diptera: Culicidae). S Asian J Trop Med Public Health 37: 915–931.
- Autran ES, Nevesb IA, da Silva CSB, GKN, Santosa CAG, da Câmarab D, Navarroa MAF (2009) Chemical composition, oviposition deterrent and larvicidal activities against Aedes aegypti of essential oils from Piper marginatum Jacq. (Piperaceae). Biores Techn 100: 2284–2288.
- Amer A, Mehlhorn H (.2006 b) Repellency effect of forty-one essential oils against Aedes, Anopheles, and Culex mosquitoes. Parasitol Res 99: 478–490.
- Prabhu K, Murugan K, Nareshkumar A, Ramasubramanian N, Bragadeeswaran S (2011) Larvicidal and repellent potential of Moringa oleifera against malarial vector, Anopheles stephensi Liston (Insecta: Diptera: Culicidae). Asian Pacific J Trop Biomed 1: 124–29.
- Gillij YG, Gleiser RM, Zygadlo JA (2008) Mosquito repellent activity of essential oils of aromatic plants growing in Argentina. Biores Techn 99: 2507–2515.
- Nerio LS, Olivero-Verbel J, Stashenko E (2010) Repellent activity of essential oils: A review. Biores Technol 101: 372–378.
- Regnault-Roger C, Vincent C, Arnason JT (2012) Essential oils in insect control: low-risk products in a high-stakes world. Annu Rev Entomol 57: 405–424. [crossref]
- Koul O, Singh R, Kaur B, Kanda D (2013) Comparative study on the behavioral response and acute toxicity of some essential oil compounds and their binary mixtures to larvae of Helicoverpa armigera, Spodoptera litura and Chilo partellus. Ind Crops Prod 49: 428–436.