Abstract
Background: Transient elastography using FibroScan is a noninvasive and reliable method to assess liver stiffness. Liver stiffness is influenced not only by fibrosis but also by liver congestion, inflammation and cholestasis. This study aimed to investigate the correlation between liver stiffness, liver congestion and liver fibrosis, and to elucidate the utility of liver stiffness measurement (LSM) in patients with chronic heart failure (CHF).
Methods: We investigated 42 patients with chronic heart failure undergoing right heart catheterization (RHC) from November 2015 to November 2016.LSM was performed with FibroScan. Patients underwent right arterial pressure (RAP) measurement by RHC.
Results: LSM was 10.9 ± 12.6 kPa. RAP was 8.0 ± 5.7 mmHg, and 18 patients had RAP>8 mmHg. LSM was correlated with FIB-4 (r=0.67, p=0.002), HA (r<0.57, p<0.001) and RAP (r=0.67, p9.65kPa) was significantly associated with shorter survival (mean OS; 19.9 vs. 29.9 months, p<0.001).
Conclusion: LSM was directly influenced by liver congestion and liver fibrosis in patients with CHF. Moreover, high LSM was demonstrated to be related with worse survival in patients with CHF.
Keywords
Fibroscan, Hyaluronic acid, Right arterial pressure, Survival
Introduction
Heart failure is a pathologic condition in which impaired pumping function reduces blood flow and leads to congestion of blood and fluids in many organs. Cardiac dysfunction causes liver damage. In particular, right-sided heart failure causes liver congestion, which is known as congestive hepatopathy [1]. Chronic liver congestion progresses to liver fibrosis [2,3]. Histological examination of liver congestion shows sinusoidal engorgement, degeneration, and variable degrees of hemorrhagic necrosis. In patients with chronic or recurrent heart failure, reticulin and collagen accumulation cause liver fibrosis [4].
Despite the seriousness of this condition, only a few studies have reported the progression of liver fibrosis in congestive hepatopathy [5]. Liver biopsy is the gold standard for fibrosis identification. However, it cannot be used as a routine screening tool to detect or monitor liver fibrosis progression due to its inherent shortcomings, which include its invasive nature and the concomitant rare potential risks of bleeding and sampling variability [6].
Because of these disadvantages, several serologic markers that can evaluate the degree of hepatic fibrosis have been used. Hyaluronic acid (HA), which is a highly evolutionarily conserved glycosaminoglycan component of the extracelluar matrix, is generally used as a serum biomarker of liver fibrosis [7]. Moreover, combined assays of multiple markers to improve the predictive ability of liver fibrosis have been also been developed. Among them, fibrosis index based on four factors (FIB-4) is a non-invasive test to stage liver fibrosis in patients with co-infected human immunodeficiency virus (HIV) and hepatitis C virus (HCV) [8]. FIB-4 relies on patient age, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels, and platelet counts, which are routinely measured and available. Recently, FIB-4 has come to be applied to various liver diseases for its convenience and cost effectiveness [9,10].
In addition to laboratory tests, the method of non-invasive transient elastography (TE) for assessing liver fibrosis has been developed and widely used in the routine clinical setting. FibroScan is a rapid, non-invasive, and reproducible approach for assessing liver fibrosis by measuring liver stiffness [11,12], and has been approved for clinical use in Japan. In various liver diseases such as viral hepatitis, alcoholic liver disease and non-alcoholic fatty liver disease, liver stiffness measurement (LSM) has been found to be strongly associated with the degree of liver fibrosis [13-15]. However, LSM is considerably influenced by liver congestion, inflammation and cholestasis, independent of the degree of fibrosis [16,17]. Colli et al. showed increased LSM in most patients with acute decompensated heart failure in the absence of parenchymal liver disease [18]. Therefore, LSM can not properly reflect liver fibrosis in patients with congestive heart failure [19]. Little is known about LSM in patients with CHF.
The purposes of this study were to assess the relationship between liver stiffness, volume status and liver fibrosis in patients with chronic heart failure (CHF), and to elucidate the utility of TE in those patients.
Methods
Patient Enrollment
We prospectively investigated LSM using transient elastography in 47 patients who were admitted to our hospital with CHF and scheduled for right heart catheterization (RHC) in the Department of Cardiology of Mie University Hospital from November 2015 to November 2016. RHC was performed in patients who required accurate hemodynamic monitoring because of clinically indeterminate volume and in patients who were refractory to initial therapy. The diagnosis of CHF was made clinically based on signs and symptoms derived from patient history and examination. Exclusion criteria included history of alcohol abuse, known chronic liver disease with an etiology other than heart failure, positivity for hepatitis B surface antigen or hepatitis C antibody, severe obesity, and ascites. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s human research committee (Authorization Number 2271).
Laboratory Tests and FIB-4
Laboratory data were obtained beginning at the closest date to RHC. FIB-4 was calculated using the formula: FIB-4 = age (years) × AST (IU/l)/[platelet count (Plt) (109/l) × ALT1/2 (IU/l)].
Echocardiography
Transthoracic echocardiography was performed using AplioTM 500 (Toshiba medical systems, Tokyo, Japan). Non-invasive RAP was estimated from diameter of inferior vena and its respiratory motion.
Cardiac Catheterization
RHC was performed in the cardiac catheterization laboratory using a flow-directed pulmonary artery catheter. Pressure calibration was performed before and after pressure measurements. All readings were referenced to the midaxillary line with the patient in the supine position. Pressure measurements were determined at the end-expiratory period, with an average of 3 to 5 cycles obtained. The physician performing the cardiac catheterization was unaware of LSM results.
Liver Stiffness Measurement
LSM was measured with FibroScan (Echosens, Paris, France) in the hepatology unit of our hospital. LSM was obtained within 24 hours before or after RHC. The tip of the probe transducer was placed on the skin between intercostal spaces and the level of the right lobe of the liver. The measurement depth was between 25 and 65 mm below the skin surface. Ten validated measurements were performed on each patient with success rates of at least 60%. The results were expressed in kilopascals (kPa). The only procedures considered reliable were those with at least 10 validated measurements and interquartile range <30% of the median value.
Statistics Analysis
Results are presented as the mean ± standard error of the mean or n, as appropriate. The means or percentages were compared by using independent Student’s t-test or the Mann-Whitney U test for continuous variables. Relationships between variables were determined using the two-sided Pearson’s correlation coefficient. Receiver operator characteristic (ROC) curves and the corresponding area under the curve (AUC) were used to obtain cut-offs for the outcomes. The Youden index was applied to calculate the optimal cutoff point. Overall survival (OS) was measured using the Kaplan-Meier method and compared using the log-rank test. Differences were considered significant at p.
Results
Patient Characteristics
In total, 47 patients were screened for this study. Five patients were excluded before scanning: 5 patients with history of alcoholic abuse, and one patient with positivity for hepatitis C antibody. A total of 42 patients (27 men and 15 women) were enrolled in this study. The distribution of the individual forms of cardiac disease is shown in Table 1. The enrolled patients represented a wide spectrum of cardiac disease. The most dominant form was valvular heart disease. The subjects’ baseline clinical and laboratory characteristics are shown in Table 2. The average duration of heart disease was 79.8 ± 85.7 months. The mean FIB-4 was 2.77 ± 1.72. In the individual components of FIB-4, the mean values were as follows: age (70.0 ± 13.9 yrs), AST (25.6 ± 9.3 IU/l), ALT (17.8 ± 8.9 IU/l), and Plt (205 ± 86 109/l). Aminotransferase levels were only slightly elevated in 2 patients. Mean HA was 102 ± 104 ng/ml, and 25 patients had abnormal HA (>50 ng/ml). In echocardiograph, the mean IVC diameter was 20.5 ± 16.4 mm. IVC did not collapse in only 4 patients. In RHC indications, the mean RAP was 8.0 ± 5.7 mmHg. Successful LSM was obtained in 42 patients. The median IQR was 0.9 and the median IQR/median of liver measurement was 20%, showing reliable results. The mean LSM was 10.9 ± 12.6 kPa, and 28 patients with chronic heart failure were higher than the normal range reported previously (normal, <5.5 kPa).
Table 1: Clinical diagnoses.
| Clinical Diagnosis |
n=42 |
| Valvular heart disease |
18 |
| Ischemic cardiomyopathy |
8 |
| Dilated cardiomyopathy |
5 |
| Cardiac sarcoidosis |
4 |
| Atrial septal defect |
3 |
| Pulmonary hypertension |
2 |
|
HF with preserved ejection fraction |
1 |
| Hypertrophic cardiomyopathy |
1 |
HF, heart failure
Table 2: Clinical and laboratory characteristics.
| Variable |
n=42 |
| Age (years) |
70.0 ± 13.9 |
| Sex (F/M) |
15/27 |
| Body mass index (kg/m2) |
22.3 ± 6.0 |
| Duration of heart disease (months) |
79.8 ± 85.7 |
| Laboratory tests | |
| Alb (mg/dl) |
3.9 ± 0.5 |
| T-Bil (mg/dl) |
0.8 ± 0.3 |
| AST (IU/l) |
25.6 ± 9.3 |
| ALT (IU/l) |
17.8 ± 8.9 |
| ALP (IU/l) |
239.0 ± 76.6 |
| BUN (mg/dl) |
22.5 ± 11.3 |
| Cre (mg/dl) |
1.07 ± 0.38 |
| WBC |
6055 ± 1718 |
| Hb |
12.8 ± 2.2 |
| Plt (109/l) |
205 ± 86 |
| BNP (pg/ml) |
253 ± 326 |
| HA (ng/ml) |
102 ± 104 |
| FIB-4 |
2.77 ± 1.72 |
| Echocardiography | |
| Ejection fraction (%) |
51.8 ± 19.5 |
| IVC diameter (mm) |
20.5 ± 16.4 |
| Hemodynamics | |
| Systolic aortic pressure (mmHg) |
123.5 ± 20.7 |
| Diastolic aortic pressure (mmHg) |
68.0 ± 11.8 |
| Heart rate (beats/min) |
76 ± 13 |
| Pulmonary capillary wedge pressure (mmHg) |
14.7 ± 8.4 |
| Mean pulmonary artery pressure (mmHg) |
22.2 ± 11.5 |
|
Mean RAP (mmHg) |
8.0 ± 5.7 |
| Cardiac index (l/min/m2) |
2.83 ± 0.84 |
| Transient elastography | |
| LSM (kPa) |
10.9 ± 12.6 |
| Interquartile range/median |
15 ± 6 |
Alb, albumen; T-Bil, total bilirubin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; WBC, white blood cells count; Hb, hemoglobin; Plt, platelet count; BUN, blood Urea nitrogen; Cre, creatinine ; Plt, platelet count; BNP, brain natriuretic peptide; HA, hyaluronic acid; FIB-4, fibrosis index based on four factors; IVC, inferior vena cava; RAP, right arterial pressure; LSM, liver stiffness measurement.
Correlation between LSM, FIB-4, HA and RAP
LSM was correlated with FIB-4 (r=0.57, p<0.001, Figure 1A), HA (r=0.67, p<0.001, Figure 1B) and RAP (r=0.67, p<0.001, Figure 1C). FIB-4 was correlated with HA (r=0.68, p<0.001, Figure 1D).
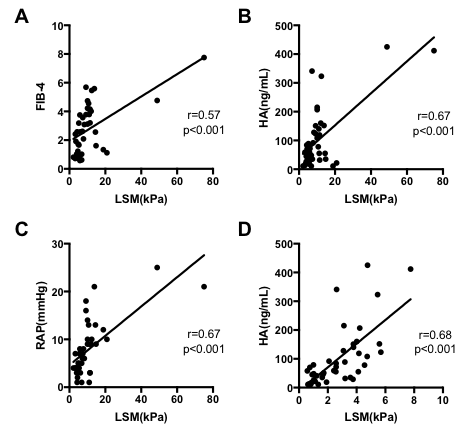
Figure 1: LSM was correlated with fibrotic markers and RAP. Correlation of LSM with FIB-4 (A). Correlation of LSM with HA (B). Correlation of LSM with RAP (C). Correlation of FIB-4 with HA (D). LSM, liver stiffness measurement; FIB-4, fibrosis index based on four factors; HA, hyaluronic acid; RAP, right arterial pressure.
Patient Outcome
Our study period had 8 out of 42 patients (19%) who died in the average follow-up period of 30.0 months. The cause of death for 7 patients included the following conditions related to CHF: heart failure, respiratory failure, and sudden death. One patient died of lymphoma. ROC analyses concerning predictors of survival yielded AUC values of 0.745 for LSM (p=0.04), 0.575 or RAP (p=0.511) (Figure 2A). We calculated the cut-off value of LSM, 9.65 (sensitivity: 0.625, and specificity: 0.647), from our ROC analysis of survival curves. Mean OS was significantly longer in patients with LSM≥9.65 versus those patients with LSM<9.65 (mean OS; 19.9 vs. 29.9 months, p<0.001; Figure 2B). Factors associated with LSM higher than 9.65 kPa are outlined in Table 3. Patients with low LSM were significantly younger (p=0.034) and had lower duration of heart disease, BNP, HA, FIB-4 and RAP but higher hemoglobin and Plt. However, Albumen, T-Bil, AST, ALT and IVC diameter had no significant differences.
Table 3: Characteristics of subjects stratified by LSM.
| Variable |
LSM>9.65kPa (n=17) |
LSM≤9.65kPa
(n=25) |
p value |
| Age (years) |
75.1 ± 10.2 |
66.6 ± 15.3 |
0.037 |
| Duration of heart disease (months) |
123 ± 100 |
50 ± 61 |
0.013 |
| Alb (mg/dl) |
3.8 ± 0.6 |
4.0 ± 0.4 |
0.394 |
| T-Bil (mg/dl) |
0.9 ± 0.3 |
0.8 ± 0.3 |
0.142 |
| AST (IU/l) |
26.6 ± 7.9 |
24.9 ± 10.3 |
0.543 |
| ALT (IU/l) |
17.2 ± 10.0 |
18.2 ± 8.3 |
0.746 |
| ALP (IU/l) |
259 ± 65 |
225 ± 82 |
0.146 |
| BUN (mg/dl) |
25.2 ± 12.0 |
20.7 ± 10.7 |
0.216 |
| Cre (mg/dl) |
1.18 ± 0.40 |
0.99 ± 0.36 |
0.131 |
| WBC |
5728 ± 2086 |
6278 ± 1420 |
0.352 |
| Hb |
11.7 ± 2.1 |
13.5 ± 2.1 |
0.011 |
| Plt (109/l) |
156 ± 68 |
239 ± 82 |
0.001 |
| BNP (pg/ml) |
418 ± 423 |
141 ± 172 |
0.019 |
| HA (ng/ml) |
150 ± 130 |
70 ± 68 |
0.029 |
| FIB-4 |
3.89 ± 1.67 |
2.01 ± 1.30 |
<0.001 |
| IVC diameter (mm) |
20.5 ± 5.3 |
20.5 ± 21.0 |
0.998 |
| RAP (mmHg) |
11.3 ± 6.4 |
5.7 ± 3.9 |
0.004 |
LSM, liver stiffness measurement; Alb, albumen; T-Bil, total bilirubin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BUN, blood Urea nitrogen; Cre, creatinine ; WBC, white blood cells count; Hb, hemoglobin; Plt, platelet count; BNP, brain natriuretic peptide; HA, hyaluronic acid; FIB-4, fibrosis index based on four factors; IVC, inferior vena cava; RAP, right arterial pressure;

Figure 2: Survival was worsened with LSM>9.65 in patients with chronic heart failure. ROC curves for identification of survival of LSM and RAP (A). Kaplan-Meier curve in patients with chronic heart failure according to LSM (B). LSM, liver stiffness measurement; ROC, receiver operating characteristic; RAP, right arterial pressure.
Discussion
In this study, there was a higher level of liver stiffness in patients with CHF without known pre-existing liver disease. The mean LSM reached 9.2 kPa in those patients, which is a significantly higher value than the normal range reported previously [20]. Several studies have investigated the influence of increased RAP in LSM during heart failure [18,21,22]. Taniguchi et al. showed a close correlation between RAP and LSM with a curvilinear regression equation in patients with heart failure [21]. We also found a good correlation between LSM and RAP in the present study, which is consistent with previous reports. The use of a non-invasive tool for evaluation of RAP should be a great interest for clinical management of patients with CHF. The evaluation of RAP has the potential to improve the hemodynamic profiling of patients, which can lead to better patient management and outcomes.
Millonig et al. showed that the central venous pressure (CVP) value reversibly controls LSM in an animal model [23]. However, Colli et al. used diuresis to demonstrate a small reduction in LSM in patients with chronic heart failure, from 8.8 to 7.2 kPa in 27 patients, with a median reduction of 1.2 kPa [18]. The explanation for the small change in LSM in patients with CHF before and after diuresis was not clear, but the elevated LSM following attainment of euvolemia may be due to the presence of the underlying fibrosis. In the present study, we found that FIB-4 index was increased in patients with CHF. FIB-4 was important biomarkers of liver fibrosis in patients with CHF.
Although little is known about the mechanism of liver fibrosis in patients with CHF, the pathogenesis of congestive hepatic fibrosis is thought to be a reaction of stellate cells following prolonged congestive heart failure or hepatic outflow obstruction. The stellate cells are transformed into alpha-smooth muscle actin-positive myofibroblasts, and these myofibroblasts produce extracellular matrix proteins in centrilobular sinusoidal areas under congestive condition [24]. Fujimoto et al. showed that HA was increased in right heart failure rat model as well as in patients with liver cirrhosis [25]. In the present study, we found that HA was increased in patients with CHF.
As mentioned, liver stiffness is influenced by liver congestion independent of the degree of liver fibrosis. In addition, inflammatory infiltration, tissue edema, and cholestasis can also affect LSM [16,17]. However, total bilirubin, AST, and ALT were within normal range in almost all the patients and there was no significant correlation between LSM and these laboratory parameters in the present study.
Previous studies demonstrated LSM is associated with risk of decompensation, liver cancer, and death in patients with chronic liver disease [26]. In the present study, we found that LSM was also associated with OS in patients with CHF. Moreover, our results showed that LSM was a better prognostic indicator than RAP for OS of CHF patients. The reason is that LSM could be affected not only by RAP but also by liver fibrosis.
There were several limitations in the present study. Firstly, the liver histological data, which would lead to a better proof of the utility of LSM, was insufficient. However, liver biopsy is invasive and considered inappropriate for patients with CHF because of the greater possibility of bleeding complications. Secondly, it is not clear whether the FIB-4 is valuable in patients with CHF. Following congestive heart failure, AST levels also may be routinely increased; however, the liver test abnormalities in our patients were very small, indicating that liver injuries were not severe in our patients. Thirdly, the sample size was not large enough for definitive conclusions. Therefore, further multicenter studies are needed to confirm the present results. Despite these limitations, our findings could be relevant in the future and will stimulate further research in this field.
In conclusion, we suggest the possibility that LSM obtained via Fibroscan may be associated with RAP, but also with the underlying liver fibrosis and survival in patients with CHF. LSM might be used in risk stratification in patients with CHF.
Author Contributions
Concept of the study (K.S, Y.T.), extraction of data (Y.T., K.S.), drafting of the manuscript (Y.T.), writing of the manuscript (Y.T., K.S., K.D), revision for important intellectual content (all authors). All authors approved the final version of the manuscript.
Funding: No external financial support was received.
Conflict of interest: Nothing to declare for all authors.
References
- Kubo SH, Walter BA, John DH, Clark M, et al. (1987) Liver function abnormalities in chronic heart failure. Influence of systemic hemodynamics. Arch Intern Med 147(7): 1227-30. Epub 1987/07/01. [crossref]
- Sherlock S (1951) The liver in heart failure; relation of anatomical, functional, and circulatory changes. Br Heart J 13(3): 273-93. Epub 1951/07/01. [crossref]
- Gelow JM, Desai AS, Hochberg CP, Glickman JN, et al. (2010) Clinical predictors of hepatic fibrosis in chronic advanced heart failure. Circ Heart Fail 3(1): 59-64. Epub 2009/10/16. [crossref]
- Myers RP, Cerini R, Sayegh R, Moreau R, et al. (2003) Cardiac hepatopathy: clinical, hemodynamic, and histologic characteristics and correlations. Hepatology 37(2): 393-400. Epub 2003/01/24. [crossref]
- Simonetto DA, Yang HY, Yin M, de Assuncao TM, et al. (2015) Chronic passive venous congestion drives hepatic fibrogenesis via sinusoidal thrombosis and mechanical forces. Hepatology 61(2): 648-59. Epub 2014/08/22. [crossref]
- Cadranel JF, Rufat P, Degos F (2000) Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology 32(3): 477-81. Epub 2000/08/29. [crossref]
- Suzuki A, Angulo P, Lymp J, Li D, et al. (2005) Hyaluronic acid, an accurate serum marker for severe hepatic fibrosis in patients with non-alcoholic fatty liver disease. Liver Int 25(4): 779-86. Epub 2005/07/07. [crossref]
- Sterling RK, Lissen E, Clumeck N, Sola R, et al. (2006) Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 43(6): 1317-25. Epub 2006/05/27. [crossref]
- Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, et al. (2007) FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 46(1): 32-6. Epub 2007/06/15. [crossref]
- Shah AG, Lydecker A, Murray K, Tetri BN, et al. (2009) Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 7(10): 1104-12. Epub 2009/06/16. [crossref]
- Martinez SM, Crespo G, Navasa M, Forns X (2011) Noninvasive assessment of liver fibrosis. Hepatology 53(1): 325-35. Epub 2011/01/22. [crossref]
- Castera L, Forns X, Alberti A (2008) Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol 48(5): 835-47. Epub 2008/03/13. [crossref]
- Castera L, Vergniol J, Foucher J, Le Bail B, et al. (2005) Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 128(2): 343-50. Epub 2005/02/03. [crossref]
- Fernandez M, Trepo E, Degre D, Gustot T, et al. (2015) Transient elastography using Fibroscan is the most reliable noninvasive method for the diagnosis of advanced fibrosis and cirrhosis in alcoholic liver disease. Eur J Gastroenterol Hepatol 27(9): 1074-9. Epub 2015/05/27. [crossref]
- Hashemi SA, Alavian SM, Gholami-Fesharaki M (2016) Assessment of transient elastography (FibroScan) for diagnosis of fibrosis in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Caspian J Intern Med 7(4): 242-52. Epub 2016/12/22. [crossref]
- Arena U, Vizzutti F, Corti G, Ambu S, et al. (2008) Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology 47(2): 380-4. Epub 2007/12/21. [crossref]
- Millonig G, Reimann FM, Friedrich S, Fonouni H, et al. (2008) Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology 48(5): 1718-23. Epub 2008/10/07.
- Colli A, Pozzoni P, Berzuini A, Gerosa A, et al. (2010) Decompensated chronic heart failure: increased liver stiffness measured by means of transient elastography. Radiology 257(3): 872-8. Epub 2010/10/12. [crossref]
- Lebray P, Varnous S, Charlotte F, Varaut A, et al. (2008) Liver stiffness is an unreliable marker of liver fibrosis in patients with cardiac insufficiency. Hepatology 48(6): 2089. Epub 2008/11/13.
- Alsebaey A, Allam N, Alswat K, Waked I (2015) Normal liver stiffness: A study in living donors with normal liver histology. World J Hepatol 7(8): 1149-53. Epub 2015/06/09. [crossref]
- Taniguchi T, Sakata Y, Ohtani T, Mizote I, et al. (2014) Usefulness of transient elastography for noninvasive and reliable estimation of right-sided filling pressure in heart failure. Am J Cardiol 113(3): 552-8. Epub 2013/12/10. [crossref]
- Hopper I, Kemp W, Porapakkham P, Sata Y, et al. (2012) Impact of heart failure and changes to volume status on liver stiffness: non-invasive assessment using transient elastography. Eur J Heart Fail 14(6): 621-7. Epub 2012/04/24. [crossref]
- Millonig G, Friedrich S, Adolf S, Fonouni H, et al. (2010) Liver stiffness is directly influenced by central venous pressure. J Hepatol 52(2): 206-10. Epub 2009/12/22. [crossref]
- Seki E, Schwabe RF (2015) Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology 61(3): 1066-79. Epub 2014/07/30. [crossref]
- Fujimoto Y, Urashima T, Shimura D, Ito R, et al. (2016) Low Cardiac Output Leads Hepatic Fibrosis in Right Heart Failure Model Rats. PLoS One 11(2): e0148666. Epub 2016/02/11. [crossref]
- Singh S, Fujii LL, Murad MH, Wang Z, et al. (2013) Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 11(12): 1573-84 e1-2; quiz e88-9. Epub 2013/08/21. [crossref]