Abstract
L-Ornithine L-aspartate (LOLA) is a 1:1 stable salt of naturally-occurring amino acids L-ornithine and L-aspartic acid. Following oral administration, LOLA is rapidly absorbed dependent on the Na + ion gradient. The elimination half-life is estimated to be in the 30–45 min range with bioavailability of 82.2%. LOLA has the proven capacity to cause lowering of blood ammonia and it does so as a result of multiple established mechanisms. Being a urea cycle intermediate and, more specifically, an activator of carbomyl phosphate synthetase, L-ornithine stimulates ammonia removal as urea by periportal hepatocytes. Both L-ornithine and L-aspartate are substrates for transamination reactions resulting in formation of glutamate, the obligate substrate for glutamine synthetase located in perivenous hepatocytes, skeletal muscle and brain. Increases of brain glutamine correlate with severity of Hepatic Encephalopathy (HE) in patients with cirrhosis. In cirrhosis, the normal pattern of inter-organ trafficking is modified and skeletal muscle replaces the liver as the major ammonia-removing organ. Muscle wasting (sarcopenia) occurs in cirrhosis as a result of exposure to ammonia and this seriously limits its ammonia-lowering capacity lead into a vicious cycle and worsening of hyperammonemia. Trials demonstrate that treatment with LOLA improves muscle function in patients with cirrhosis. There is also evidence to suggest that LOLA also has direct hepato-protective actions in these patients via mechanisms related to the production of antioxidants and the synthesis of nitric oxide leading to improved hepatic microcirculation. Over 20 randomized controlled trials together with systematic analyses with meta-analyses have demonstrated that LOLA is effective for the prevention and treatment of HE in cirrhosis where improvements in mental state occurred as a consequence of the lowering of circulating ammonia.
Keywords
L-Ornithine L-Aspartate, LOLA, Ammonia, Hyperammonemia, Cirrhosis, Hepatic Encephalopathy, Muscle, Sarcopenia, Meta-analysis
Introduction
The ammonia molecule exists in biological systems as an equilibrium between ammonia gas (NH3) and the ammonium ion (NH4)+ dependent upon pH so that, at normal physiological pH, 96% of ammonia is in the ionic form and blood ammonia concentrations are in the 30–50uM range.
A key function of the liver is the removal of excess blood-borne ammonia generated primarily from protein digestion in the intestines and carried to the liver via the portal vein. Hepatic ammonia detoxification occurs by two mechanisms as a function of the identity of the liver cell. In humans, the sites for the synthesis of urea and glutamine are differentially located in the liver acinus. Incorporation ammonia into the molecule of urea takes place in periportal hepatocytes that are known to express genes associated with constituent enzymes of the urea cycle. Scavenging of remaining ammonia then occurs by incorporation into the molecule of glutamine by perivenous hepatocytes expressing the gene coding for Glutamine Synthetase (GS) [1]. Location of these important enzymatic steps in relation to inter-organ trafficking of ammonia between the intestines, liver, skeletal muscle and brain is depicted in a simplified schematic form in Figure 1A.
In chronic liver disease, loss of hepatic parenchyma results in increases in vascular resistance and portal hypertension leading to portal-systemic shunting of ammonia-rich venous blood. Concomitantly, a significant loss of up to 85% of functional periportal and perivenous hepatocytes occurs resulting in severe impairments of hepatic ammonia detoxification (Figure 1B).
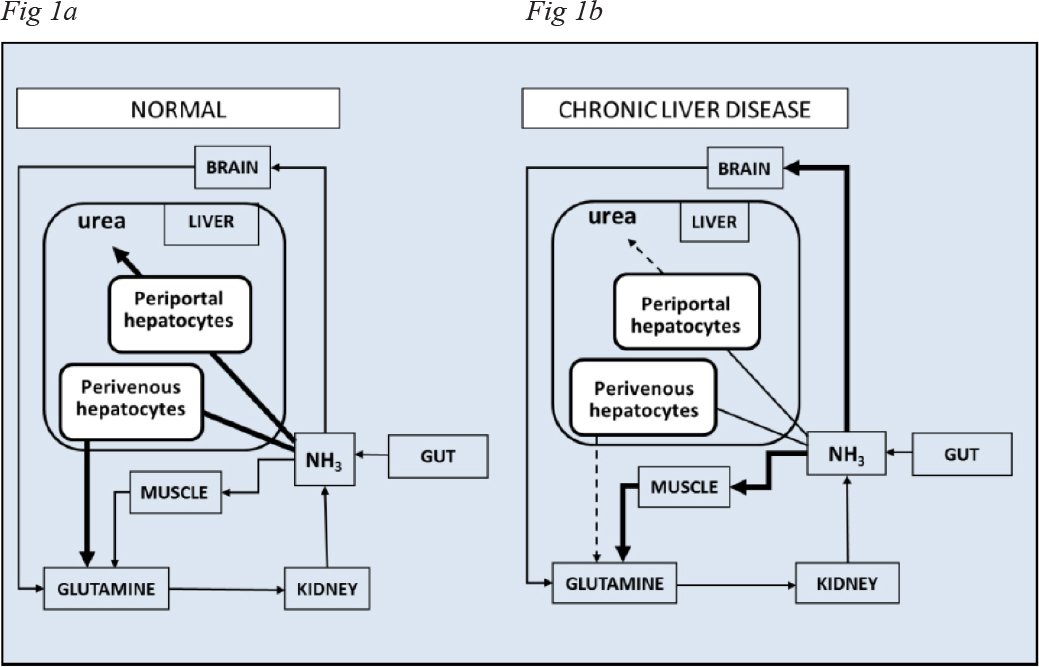
Figure 1. Simplified schematic representation of the steps involved in inter-organ trafficking of ammonia between the gut, liver, skeletal muscle, brain and kidney in A: normal individuals compared to B: patients with chronic liver disease and HE.
Recent studies using neuroimaging and spectroscopic techniques confirm the long-held view that ammonia plays a key role in the pathogenesis of Hepatic Encephalopathy (HE) in cirrhosis and, consequently, ammonia-lowering strategies remain the mainstay for the prevention and treatment of HE. Such treatments fall into one of two general types namely those aimed at the reduction of ammonia absorption from the gastrointestinal tract (non-absorbable disaccharides, probiotics and antibiotics) and those aimed at ammonia removal. L-Ornithine L-Aspartate (LOLA) belongs to the latter category.
Pharmacokinetics/Pharmacodynamics of LOLA
LOLA is a 1:1 stable salt of the naturally-occurring amino acids L-ornithine and L-aspartic acid. Orally-administered LOLA is rapidly absorbed by active transport across the brush border of the intestinal epithelium largely dependent on the Na+ ion gradient [2]. L-aspartate is transported by the dicarboxylic amino acid transporter. In the upper gut, LOLA is readily cleaved into its constituent amino acids. The elimination half-life of the constituent amino acids of LOLA has been estimated to be relatively short, in the 30–45 min range with a bioavailability of 82.2% following either oral or intravenous administration.
Mechanisms Responsible for the Ammonia-Lowering Actions of LOLA
Optimization of Ammonia-Removing Metabolic Pathways in Residual Periportal and Perivenous Hepatocytes
Results of studies in isolated hepatocytes have established that urea synthesis from ammonia is limited by the supply of L-ornithine and that L-ornithine requirements for the synthesis of urea are increased as a function of the supply of ammonia [3].
LOLA removes ammonia by supplying L-ornithine , a urea cycle intermediate and activator of the enzyme carbamoyl phosphate synthetase (Figure 2A) leading to increased synthesis of urea and this occurs in cirrhosis in the residual 15–20% of functional periportal hepatocytes.
Both L-ornithine and L-aspartate are substrates for transamination reactions (Figures 2B, 2C) both of which result in increased synthesis of L-glutamate, the obligate substrate for GS (Figure 2D) that is located in perivenious hepatocytes as well as in skeletal muscle and in brain and increased flux through GS in these organs leads to increased glutamine production in patients with cirrhosis and HE where increased brain glutamine signals from Magnetic Resonance Spectroscopic studies are predictors of HE grade in these patients [4].
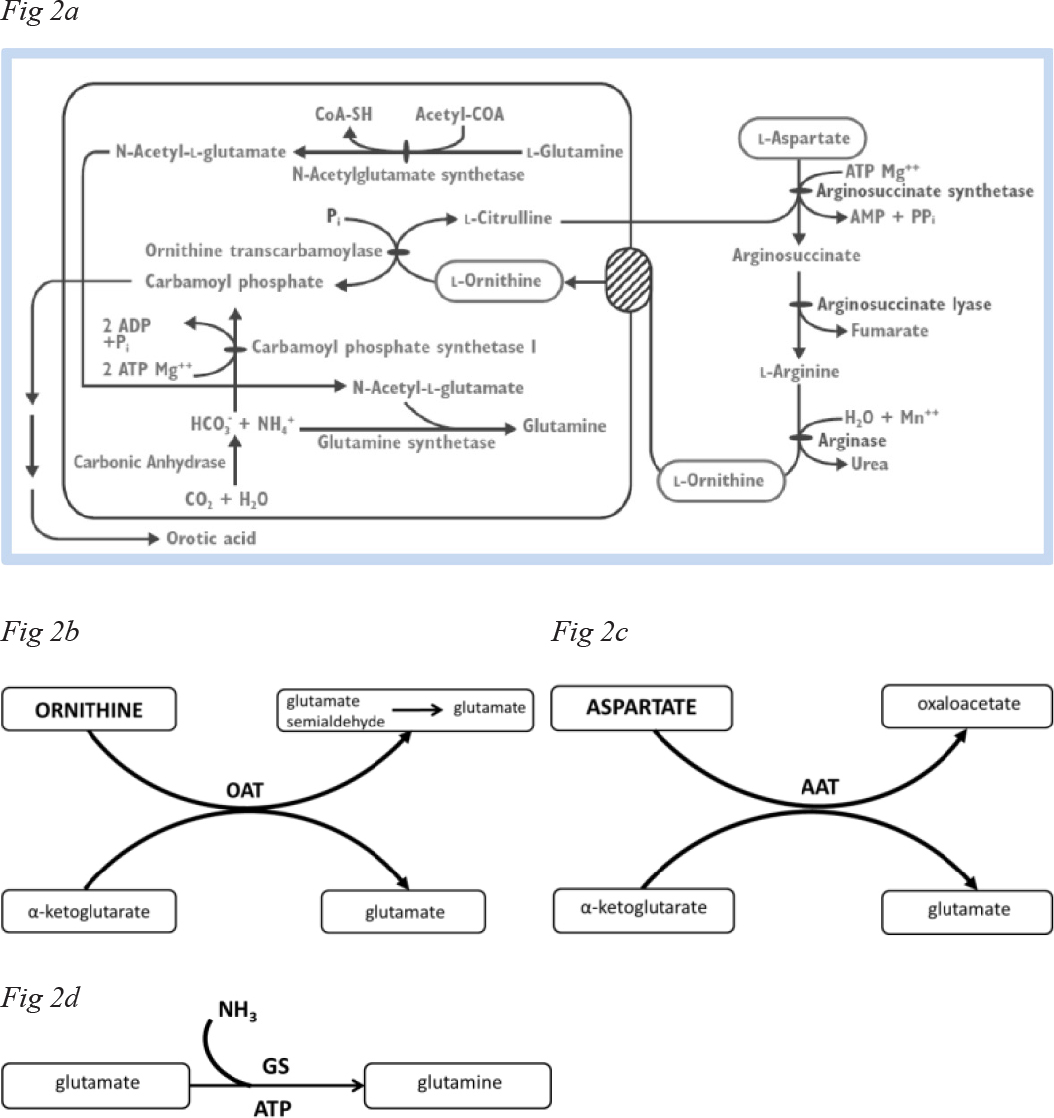
Figure 2. Metabolic conversions of L-ornithine and L-aspartate via A: elements of the urea cycle, B: ornithine aminotransferase (OAT), C: aspartate aminotransferase (AAT), D: glutamine synthesis (GS).
In a randomized double-blind, placebo-controlled trial, 10 patients with cirrhosis and hyperammonemia were treated with infusions of LOLA (5–40g over 8h). Venous blood ammonia concentrations were lowered in a dose-dependent manner compared to placebo [3].
Prevention of Sarcopenia and Stimulation of Ammonia Removal by Skeletal Muscle
Under normal physiological conditions, skeletal muscle plays a minor role in the process of ammonia removal. However, studies of Arterio-Venous (A-V) differences across the forearm of patients with cirrhosis reveal significant increases of the fractional extraction of ammonia with concomitantly increased release of glutamine [5]. These findings were subsequently confirmed in a study of the dynamics of ammonia metabolism in patients with cirrhosis using 13NH3 Positron Emission Tomography in which increased trapping of ammonia was observed [6].
Studies in experimental animal models of chronic liver disease suggest that the mechanism responsible for the activation of the skeletal muscle pathway for ammonia removal in cirrhosis is underpinned by a post-translational induction of the GS gene [7] but increases in expression of ammonia transporters could also be implicated [8].
Clearly, the physiological and functional integrity of skeletal muscle represents a potential limitation on its capacity to remove blood-borne ammonia and in cirrhosis severe muscle wasting (sarcopenia) is a common complication that is associated with increased mortality and poor post-transplant outcomes [9]. Notably, the fractional extraction of ammonia is significantly decreased in sarcopenic (compared to non-sarcopenic) patients with cirrhosis [5] resulting in the aggravation of hyperammonemia.
To make matters worse, there is emerging evidence to suggest that sarcopenia in cirrhosis is the consequence of exposure of the muscle to ammonia itself [10]. Evidence for this includes the results of in vitro studies and in studies in an experimental animal model of chronic liver disease. For example, exposure of differentiated myotube preparations to ammonia leads to decreases of myotube diameters and protein synthesis as well as increased expression of autophagy markers [11]. Portacaval anastomosis (PCA) in the rat resulted in reduced muscle mass, muscle fibre diameter and grip strength as a function of increases in muscle and blood ammonia.
Based upon the above reports it was suggested that a “vicious cycle” occurs in chronic liver disease whereby hyperammonemia attributed to its decreased hepatic removal leads to muscle dysmetabolism and autophagy typical of sarcopenia which, in turn limits the capacity of skeletal muscle to fulfil its task as alternative pathway for ammonia removal in the form of glutamine resulting in worsening of hyperammonemia and the cycle goes around [12]. A simplified schematic representation of the steps involved in the cycle is provided in Figure 3.
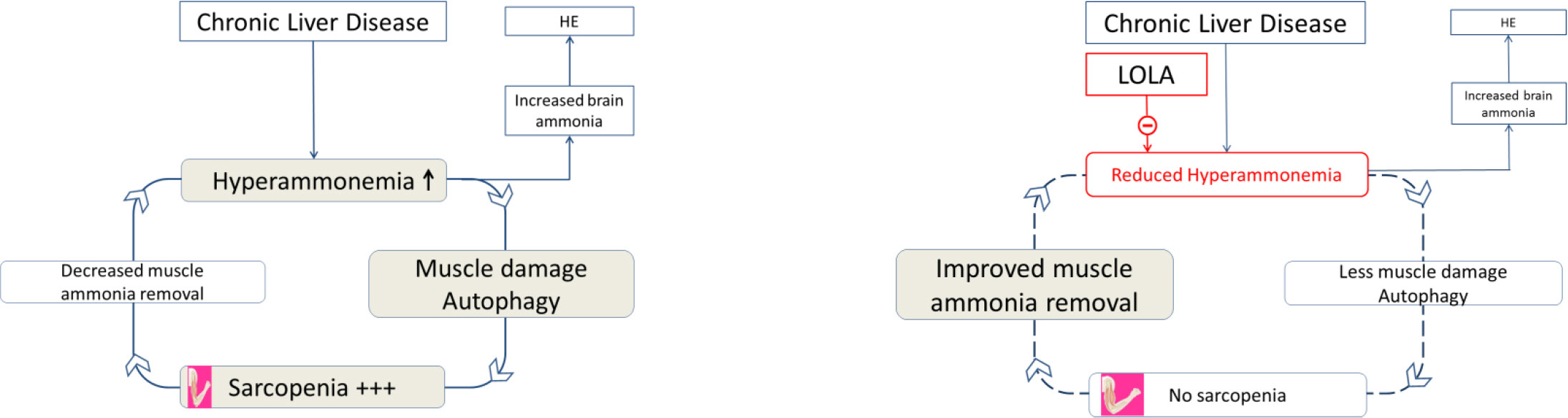
Figure 3. a. Schematic representation of the vicious cycle whereby hyperammonemia resulting from decreased ammonia removal by the liver leads to muscle damage/autophagy and sarcopenia. Sarcopenia results in a serious diminution of the capacity of muscle to remove blood-borne ammonia leading to worsening of hyperammonemia and the vicious cycle continues. b. Schematic representation of the vicious cycle whereby treatment with LOLA results in the lowering of hyperammonemia by multiple mechanisms described in the text which, in turn, relieves the damage to skeletal muscle/sarcopenia, the muscle’s capacity to remove blood-borne ammonia is restored.
LOLA is commonly employed for the treatment of HE in cirrhosis by virtue of its efficacy for lowering of blood ammonia as summarized in a systematic review and meta-analysis [13]. Making use of the PCA rat model of chronic liver failure described above with LOLA and rifaximin results in significant improvements in skeletal muscle mass and muscle fibre diameters as well as grip strength and muscle protein synthesis rates as a function of reduced concentrations of both blood and skeletal muscle concentrations of ammonia [12].
These results add to a growing body of evidence suggesting a role for LOLA in the prevention and treatment of sarcopenia in cirrhosis. More direct evidence is provided by the results of a trial in 16 patients with cirrhosis-related sarcopenia who were randomized to receive LOLA or placebo. Muscle protein synthesis rates in biopsies of anterior tibalis muscle improved markedly in the LOLA treatment group who also manifested such improvements in response to feeding [14].
Direct Hepatoprotective Properties of LOLA
This area of research was founded following the publication of reports of improvements in liver enzymes, total bilirubin and improved mental state following treatment with a large range of doses of the oral formulation of LOLA in large cohorts of patients with fatty liver or cirrhosis [15, 16]. Although uncontrolled and observational in nature, these reports were the first to suggest beneficial effects of LOLA on both liver function and severity of HE in chronic liver diseases. Hepatoprotective properties of LOLA in cirrhosis were subsequently confirmed in Randomized Controlled Trials (RCTs) in patients with cirrhosis and a range of subtypes and degrees of severity of HE where either intravenous or oral formulations of LOLA were found to be effective. Some examples of these trials are:
In an RCT of 120 patients with cirrhosis of predominantly non-alcoholic etiology and mild-to-severe overt HE, treatment with intravenous LOLA (20g/d, 3days) resulted in lowering of blood ammonia, improvements in HE severity and decreases of serum bilirubin together with improvements in Prothrombin Time (PT) [17] suggesting that improved liver function played a significant role. In fact, multivariate analysis showed that Improvement in PT was an independent factor associated with improvement of mental state in grades II-IV HE.
In an RCT of 64 patients with cirrhosis and minimal HE treated with oral LOLA (5g/d tid, 60 days), all showed improvements in psychometric test scores and a slowing of progression to overt HE six months post-treatment [18]. In this trial, significant improvements in Child-Pugh and MELD scores in patients receiving LOLA led the authors to conclude that the lowering of blood ammonia and delayed progression of MHE to OHE was the consequence of improvements in hepatic function. It has been suggested that improvements in MELD scores following treatment with LOLA could have a potential positive impact on liver transplant priority and outcomes [9]. A recent systematic review with meta-analysis demonstrated that LOLA was effective in patients with cirrhosis for OHE prevention and prophylaxis over a range of clinical presentations [19].
In an RCT of 40 patients with cirrhosis having received successful TIPSS placements treated with intravenous LOLA (30g/d, 7 days), lowering of blood ammonia along with improvements in mental state were observed on days 1, 4 and 7 post-TIPSS as well as a slower progression of MHE to OHE. [20] These beneficial effects were accompanied by lowering of blood transaminases and bilirubin together with stabilization of MELD scores. It was suggested that a 7-day prophylactic use of intravenous LOLA would be sufficient to alleviate hepatocellular damage due to TIPSS.
Role of anti-oxidants
Studies of the potential mechanisms responsible for the hepatoprotective properties of LOLA are few in number and are currently focused on the synthesis of agents derived from the conversion of the constituent amino acids of LOLA to agents with established anti-oxidant properties such as glutathione (GSH) and glutamine [21].
Conversion of L-ornithine or L-aspartate to glutamate occurs by way of metabolic conversions depicted in Figures 2A, B above and the synthesis of glutamine from glutamate occurs readily in liver and skeletal muscle via the enzyme GS. There has been a recent upsurge of interest in the role of glutamine in anti-oxidant pathways in general and as a hepato-protective agent in chronic liver disease. In an experimental animal model of non-Alcoholic Fatty Liver Disease (NAFLD), oral glutamine supplementation was found to be hepato-protective via inhibition of NF-kB p65 expression and improvement of hepatic steatosis [22, 23]. The hepatoprotective effect of glutamine did not appear to be mediated via increased conversion of glutamine to GSH [22].
Synthesis of GSH from glutamate (Figure 4), cysteine and glycine is catalyzed by two enzymes g-glutamylcysteine synthetase and GSH synthetase acting in sequence [24]. In studies of HE in animals with toxic liver injury characterized by increased liver transaminases and bilirubin, levels of GSH were found to be significantly reduced [25]. Treatment with LOLA resulted in attenuation of the increased transaminases and bilirubin concomitant with normalization of GSH levels.
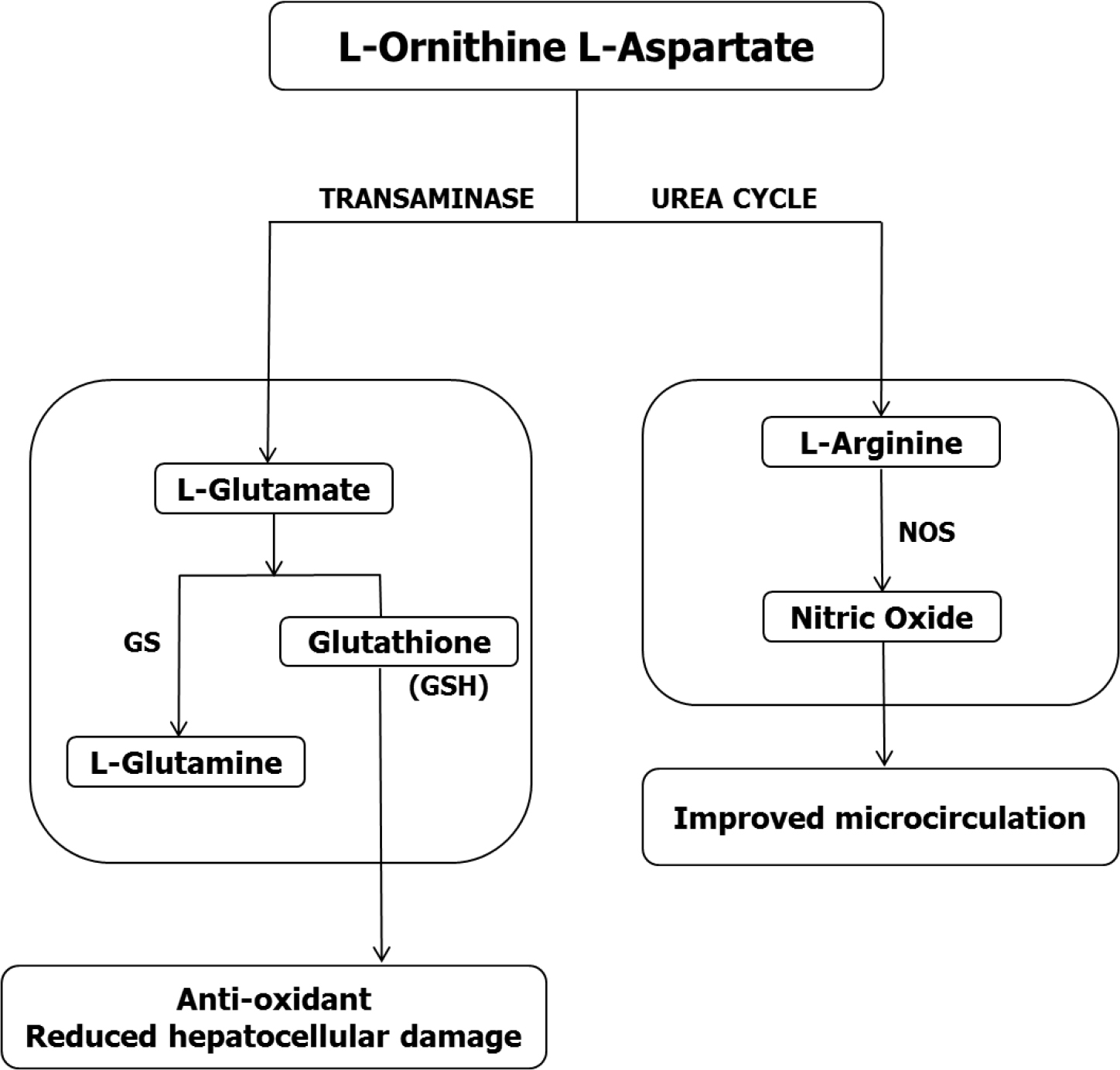
Figure 4. Possible mechanisms whereby L-ornithine L-aspartate (LOLA) exerts hepatoprotection properties mediated by the conversion of L-ornithine to L-glutamate followed by the synthesis of established antioxidants L-glutamine and glutathione (GSH). In parallel, the conversion of L-ornithine to L-arginine via the urea cycle provides the substrate for production of nitric oxide (NO) via the enzyme nitric oxide synthase (NOS) with the potential to improve hepatic microcirculation.
Role of nitric oxide
An alternative (or additional) mechanism implicated in the hepato-protective properties of LOLA involves the increased production of Nitric Oxide (NO). Studies in experimental animals and in patients with cirrhosis have consistently shown that LOLA treatment results in increased synthesis of L-arginine [3, 26] and L-arginine is the obligate substrate for Nitric Oxide Synthase (NOS) the enzyme responsible for the synthesis of NO (Figure 4). Moreover, the administration of L-arginine to animals with experimental steatosis has been shown to result in improvements in hepato-vascular perfusion [27]. Improved hepatic micro-perfusion has the potential to provide a second possible mechanism whereby LOLA treatment results in hepato-protection.
Clinical Efficacy of LOLA for The Prevention and Treatment of HE in Cirrhosis: The Evidence Based Upon the Results of Randomized Controlled Trials, Systematic Reviews and Meta-Analyses
Beneficial effects of LOLA on blood ammonia and mental state have been reported in over 20 randomized controlled clinical trials (RCTs), the findings from the majority of which have been published in peer-reviewed biomedical journals. In the 2000–2017 period results of systematic reviews with meta-analysis started to appear. [28–31] However, most of these analyses gave were performed using data from limited numbers of trials and/or were published in abstract form only resulting in limited information with respect to trial quality and risk of bias assessments that are essential for the interpretation of their findings. Moreover, the findings themselves were inconsistent with reports of efficacy of LOLA for the treatment of a range of HE presentations including MHE and OHE [30] but no such efficacy of LOLA on MHE by other investigators [29, 31]. Assessment of the effects of LOLA on blood ammonia was made in only 2 of the 4 studies, one in fasting blood samples [30], the other following post-prandial sampling [28]. No efforts were made in these studies to separately assess the efficacy of intravenous and oral formulations of LOLA on either the lowering of blood ammonia or on mental state.
In view of these inconsistent findings and generally poor quality of the studies noted above, it was not surprising that the AASLD/EASL committee responsible for the preparation of guidelines for the use of various agents for use in the treatment of HE in cirrhosis (published in 2014) expressed rather limited enthusiasm for LOLA compared to alternative agents [32]. In fact, the Guidelines Committee had managed to identify only a single RCT upon which to base their recommendations.
Consequently, a new systematic review with meta-analysis was undertaken with the objectives of assessment of the evidence base with respect to the efficacy of LOLA for the prevention and treatment of HE in cirrhosis based upon the results of RCTs [13, 33]. Efficacy was defined by two parameters; firstly, the ability of LOLA to cause significant reductions of blood ammonia and secondly, LOLA‘s effects on improvement in mental status. Subgroup analysis was used to assess efficacy in patients with MHE or OHE and for prevention of deterioration of MHE to OHE in suitably-designed trials. Efficacy of intravenous and oral formulations of LOLA was independently assessed by subgroup analysis.
Ammonia-lowering action of LOLA in Patients with Cirrhosis-Related HE
Figure 5 represents Forest plots showing the pooled effect of LOLA compared to placebo/no intervention on blood ammonia in 709 patients with cirrhosis-related HE (either MHE or OHE) [17, 18, 34–39]. LOLA was found to be consistently effective with MD of -17.50, [95% CI: -27.73 to -7.26], test for overall effect: Z = 3.35, p = 0.0008.
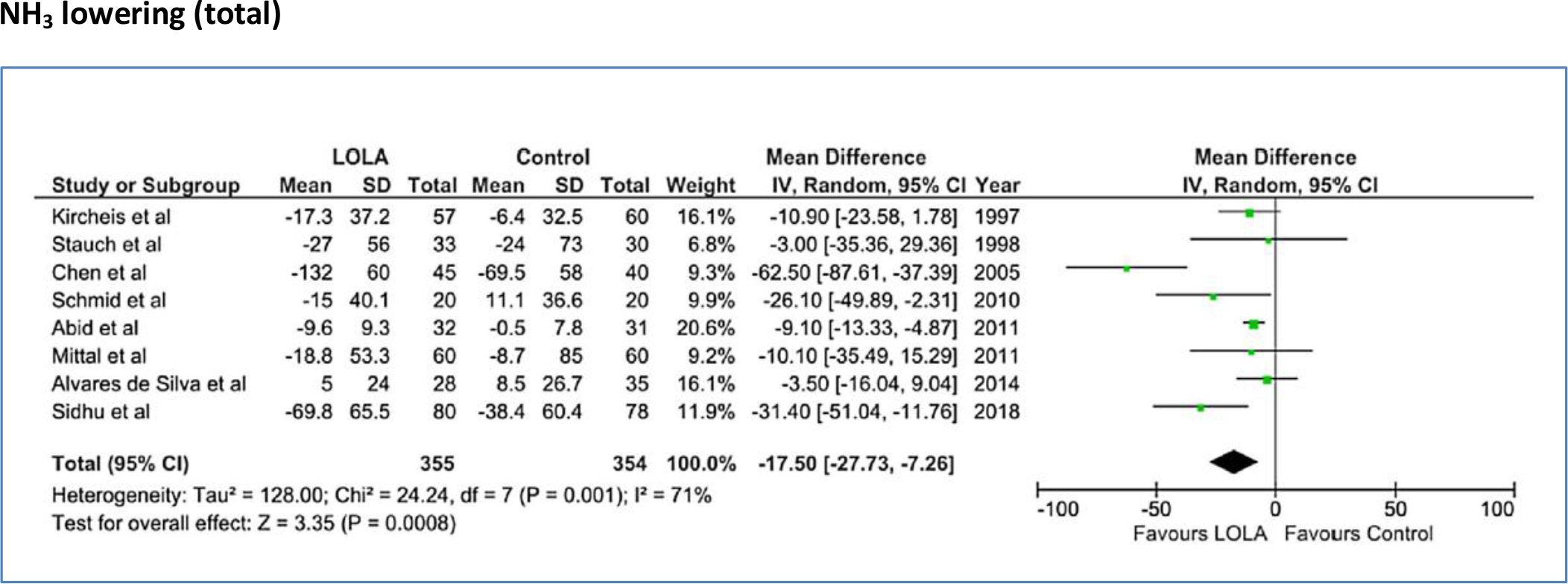
Figure 5. Forest plot indicating the pooled effect of LOLA (either oral or intravenous formulation) versus placebo/no intervention for the efficacy of lowering of hyperammonemia in patients with cirrhosis and HE. RR: Risk Ratio, CI: Confidence interval, SD: standard deviation.
Moreover, as shown by the analysis of the data in Table 1, both intravenous and oral formulations were found to be effective for lowering of blood ammonia in these trials.
Table 1. Pooled effects of intravenous (iv) or oral formulations of LOLA compared to placebo/no intervention (control) on blood ammonia concentrations in patients with cirrhosis and HE
|
Trial endpoint |
Number of patients |
MD |
95% CI |
Z score |
p-value |
|
|
LOLA |
Control |
|
|
|
|
|
|
NH3 lowering (total) |
355 |
354 |
–17.5 |
[–27.73, –7.25] |
3.35 |
0.0008 |
|
NH3 lowering (iv LOLA) |
262 |
258 |
–27.16 |
[–44.77, –9.56] |
3.02 |
0.002 |
|
NH3 lowering (oral LOLA) |
93 |
95 |
–8.44 |
[–12.42, –4.46] |
4.16 |
<0.0001 |
Effect of LOLA on Improvement of Mental State in Patients with Cirrhosis-Related HE
Figure 6 represents Forest plots indicating the pooled effect of LOLA compared to placebo/no intervention on improvement of mental state in 843 patients diagnosed with MHE or OHE according to psychometric test procedures or Westhaven criteria respectively [17, 18, 34–41]. In 8/9 trials, treatment effect favored LOLA with RR of 1.36 [95% CI: 1.10, 1.69], test for overall effect, Z = 2.82, p<0.005.
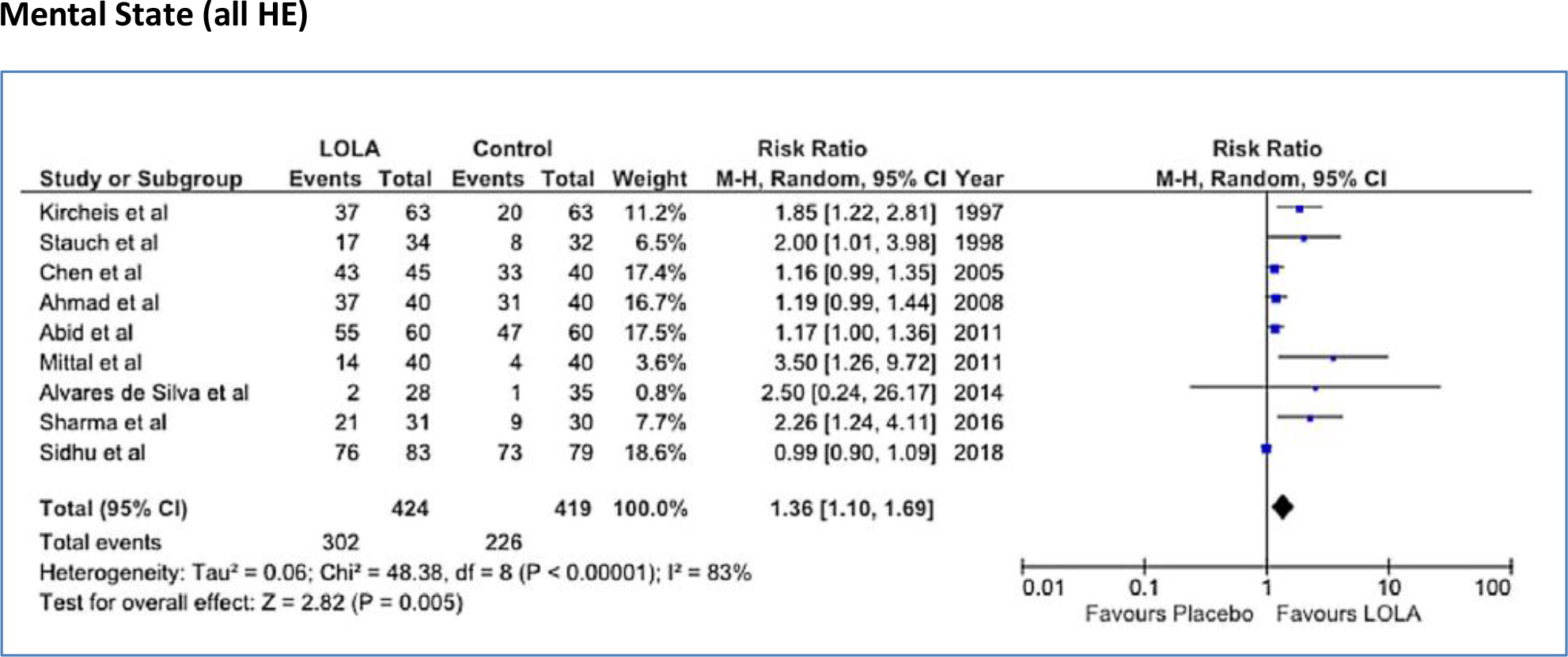
Figure 6. Forest plot indicating the pooled effect of LOLA (either oral or intravenous formulation) versus placebo/no intervention for the efficacy of improvement of mental state in patients with cirrhosis and MHE or OHE. RR: Risk Ratio, CI: Confidence Interval.
Subgroup analysis revealed that LOLA was effective for improvement of mental state in trials of patients with MHE or OHE [13] and, in the case of MHE trials, the oral formulation of LOLA (4 trials) appeared to be superior to that of the intravenous formulation (2 trials) for improvement of mental state (Table 2).
Table 2. Pooled effects of intravenous (iv) or oral formulations of LOLA compared to placebo/no intervention (control) on mental state improvement in patients with cirrhosis and HE
|
Trial endpoint |
number of patients |
RR |
95% CI |
Z score |
p-value |
|
|
LOLA |
Control |
|
|
|
|
|
|
Mental state (all HE) |
424 |
419 |
1.36 |
[1.10, 1.69] |
2.82 |
0.005 |
|
Mental state (OHE) |
282 |
269 |
1.19 |
[1.01, 1.39] |
2.14 |
0.03 |
|
Mental state (MHE) |
142 |
150 |
2.15 |
[1.48, 3.14] |
3.98 |
<0.0001 |
|
Mental state (MHE, iv) |
32 |
33 |
1.67 |
[0.90, 3.08] |
1.64 |
0.10/ns |
|
Mental state (MHE, oral) |
110 |
117 |
2.54 |
[1.54, 4.18] |
2.54 |
0.0002 |
Efficacy of LOLA Compared to other Therapeutic Agents
In a head-to-head RCT comparing LOLA with the non-metabolizeable dissaccharide lactulose, decreases in blood ammonia that were comparable in magnitude were reported [42] but only patients in the LOLA treatment arm of the trial showed significant improvements in psychometric test scores, Westhaven criteria scores, asterixis grades and EEG activity. Subsequent RCTs confirmed the comparable efficacies of LOLA with other agents including lactulose, riraximin and probiotics for improvements in psychometric test and Critical Flicker Frequency (CFF) test scores as well as for the prevention of deterioration from MHE to OHE [38, 41].
A particular type of meta-analysis known as network meta-analysis has been employed on two occasions in which the efficacy of LOLA was compared with other commonly-prescribed agents used for the treatment of HE in cirrhosis [43, 44]. In the first such analysis, only treatment with LOLA or Branched-Chain Amino Acids (BCAAs) were effective in improving OHE with trends towards improvement reported for lactulose, neomycin and rifaximin. Only LOLA treatment resulted in significant lowering of blood ammonia [43]. In a second network analysis, rifaximin, LOLA and BCAAs were found to be superior to lactulose or probiotics and LOLA was found to reduce the risk of deterioration of MHE to OHE [44].
LOLA for OHE prophylaxis and future indications
OHE occurs in up to 50% of patients with cirrhosis following the Transjugular Intrahepatic Portosystemic Stent Shunt (TIPSS) procedure for the management of complications of portal hypertension and refractory ascites. An RCT of 40 TIPSS patients demonstrated that intravenous LOLA (30g/d, 7 days) was effective for the lowering of blood ammonia leading to improvements in psychometric test scores and a slowing of progression to OHE. Moreover, in this study, improvements in liver enzymes, bilirubin and MELD scores were also reported consistent with improvements in liver function [45].
The effectiveness of LOLA for secondary OHE prophylaxis in patients with cirrhosis has been demonstrated in a double-blind RCT of 150 patients [46] in which the probability of developing OHE was reduced and the time to breakthrough of HE was significantly slowed in LOLA-treated patients compared to placebo. These benefits were accompanied by significant reductions of blood ammonia and improvements in CFF scores.
More recently, in a placebo-controlled RCT in patients with cirrhosis and variceal bleeding, treatment with LOLA or rifaximin was found to be effective in preventing the primary development of HE in these patients [46]. If confirmed, these interesting findings may herald the start of the more widespread use of LOLA for primary HE prophylaxis in patients with cirrhosis.
Funding: Financial support for research conducted in the author’s Research Unit and related publications were provided by The Canadian Institutes of Health Research.
References
- Kaiser S, Gerok W, Häussinger D (1988) Ammonia and glutamine metabolism in human liver slices: new aspects on the pathogenesis of hyperammonaemia in chronic liver disease. Eur J Clin Invest 18: 535–542.
- Kircheis, G and Lüth, S (2019) Pharmacokinetic and Pharmacodynamic Properties of L-Ornithine L-Aspartate (LOLA) in Hepatic Encephalopathy. Drugs 79: 23–29.
- Staedt U, Leweling H, Gladisch R, Kortsik C (1993) Effects of ornithine aspartate on plasma ammonia and plasma amino acids in patients with cirrhosis. A double-blind, randomized study using a four-fold crossover design. J Hepatol 19: 424–430.
- Laubenberger J, Haussinger D, Boyer S, Gufler H (1997) Protein magnetic resonance spectroscopy of brain in symptomatic and asymptomatic patients with liver cirrhosis. Gastroenterology 112: 1610–1616.
- Ganda OP, Ruderman NB (1976) Muscle nitrogen metabolism in chronic hepatic insufficiency. Metabolism 25: 427–435.
- Lockwood AH, Weissenborn K, Butterworth RF (1997) An image of the brain in patients with liver disease. Curr Opin Neurol 10: 525–533. [crossref]
- Desjardins P, Rao KV, Michalak A, Rose C (1999) Effect of portacaval anastomosis on glutamine synthetase protein and gene expression in brain, liver and skeletal muscle. Metab Brain Dis 14: 273–280.
- Takeda K, Takemasa T (2015) Expression of ammonia transporters Rhbgand RHcg inmouse skeletalmuscle and the effects of 6-week training on these proteins. Physiol Rep 3: 12596.
- Butterworth, RF (2019) L-ornithine L-aspartate for the Treatment of Sarcopenia in Cirrhosis: Potential Impact on the Outcome of Liver Transplantation. Ann Gastroenterol Dig Dis 2: 006–009.
- Qiu J, Tsien C, Thapalaya S, Narayanan A (2012) Hyperammonemia mediated autophagy in skeletal muscle contributes to sarcopenia of cirrhosis. Am J Physiol Endocrinol Metabol 303: 983–993.
- Kumar A, Davuluri G, Silva RNE, Engelen MPKJ (2017) Ammonia lowering reverses sarcopenia of cirrhosis by restoring skeletal muscle proteostasis. Hepatology 65: 2045–2058.
- Butterworth RF (2019) L-Ornithine L-Aspartate for the Treatment of Sarcopenia in Chronic Liver Disease: The Taming of a Vicious Cycle. Can J Gastroenterol Hepatol Article ID 8182195.
- Butterworth RF, Kircheis G, Hilger N, McPhail MJW (2018) Efficacy of l-ornithine l-aspartate for the treatment of hepatic encephalopathy and hyperammonemia in cirrhosis: systematic review and meta-analysis of randomized controlled trials. J Clin Exp Hepatol 8: 301–313.
- Reynolds N, Downie S, Smith K, Kircheis G (1999) Treatment with L-ornithine-L-aspartate infusion restores muscle protein synthesis responsiveness to feeding in patients with cirrhosis. J Hepatol 30: 65.
- Gruengreiff, K, Lambert-Baumann J (2001) Efficacy of L-ornithine L-aspartate granules in chronic liver diseases. MedWelt 52: 219–226.
- Butterworth RF, Gruengreiff K (2018) L-ornithine L-aspartate (LOLA) for the treatment of hepatic encephalopathy in cirrhosis: evidence for novel hepatoprotective mechanisms. J Liver Clin Res 5: 1044.
- Abid S, Jafri W, Mumtaz K, Islam M (2011) Efficacy of l-ornithine-l-aspartate as an adjuvant therapy in cirrhotic patients with hepatic encephalopathy. J Coll Phys Surg Pak 21: 666–671.
- Alvares-da-Silva MR, de Araujo A, Vicenzi JR, da Silva GV (2014) Oral L-ornithine L-aspartate in minimal hepatic encephalopathy: randomized double-blind placebo-controlled trial. Hepatol Res 44: 956–963.
- Butterworth RF (2019) Beneficial effects of L-Ornithine L-Aspartate for prevention of overt hepatic encephalopathy in patients with cirrhosis: a systematic review with meta-analysis. Metab Brain Dis under review.
- Bai M, He C, Yin Z, Niu J (2014) Randomised clinical trial: L-ornithine-L-aspartate reduces significantly the increase of venous ammonia concentration after TIPSS. Aliment Pharmacol Ther 40: 63–71.
- Butterworth RF, Canbay A (2019) Hepatoprotection by L-Ornithine L-Aspartate in Non-Alcoholic Fatty Liver Disease. Dig Dis 37: 63–68. [crossref]
- Lin Z, Cai F, Lin N, Ye J (2014) Effects of glutamine on oxidative stress and nuclear factor-?B expression in the livers of rats with non-alcoholic fatty liver disease. Exp Ther Med 7: 365–370.
- Sellmann C, Jin CJ, Degen C, De Bandt JP et al. (2015) Oral glutamine supplementation protects female mice from non-alcoholic steatohepatitis. J Nutr 145: 2280–2286.
- Wu G, Fang YZ, Yang S, Lupton JR, Turner ND (2004) Glutathione metabolism and its implications for health. J Nutr 134: 489–492. [crossref]
- Najmi AK, Pillai KK, Pal SN, Akhtar M (2010) Effect of L-ornithine L-aspartate against thioacetamide-induced hepatic damage in rats. Ind J Pharmacol 42: 384–387.
- Rose C, Michalak A, Pannunzio P, Therrien G (1998) L-Ornithine L-aspartate in experimental portal-systemic encephalopathy: therapeutic efficacy and mechanism of action. Metab Brain Dis 13: 147–157.
- Ijaz S, Yang W, Winslet MC, Seifalian AM (2005) The role of nitric oxide in the modulation of hepatic microcirculation and tissue oxygenation in an experimental animal model of hepatic steatosis. Microvasc Res 70: 129–136.
- Delcker AM, Jalan R, Schumacher M, Comes G (2000) L-ornithine-L-aspartate versus placebo in the treatment of hepatic encephalopathy: a meta-analysis of randomised placebo-controlled trials using individual data. Hepatology 4: 604.
- Jiang Q, Jiang XH, Zheng MH, Chen YP (2009) L-Ornithine-l-aspartate in the management of hepatic encephalopathy: a meta-analysis. J Gastroenterol Hepatol 24: 9–14. [crossref]
- Bai M, Yang Z, Qi X, Fan D (2013) L-Ornithine L-aspartate for hepatic encephalopathy in patients with cirrhosis: a meta-analysis of randomized controlled trials. J Gastroenterol Hepatol 28: 783–792.
- Goh ET, Stokes CS, Vilstrup H, Gluud LL (2017) L-Ornithine L-aspartate for hepatic encephalopathy: a systematic review with meta-analysis of randomised controlled trials. J Hepatol 66: 131.
- Vilstrup H, Amodio P, Bajaj J, Cordoba J (2014) Hepatic encephalopathy in chronic liver disease: 2014 practice guidelines by the American Association for the study of Liver Disease and the European Association for the Study of the Liver. Hepatology 60: 715–735.
- Butterworth RF, McPhail MJW (2019) L-Ornithine L-aspartate for hepatic encephalopathy in cirrhosis: results of randomized controlled trials and meta-analysis. Drugs 79: 31–37.
- Kircheis G, Nilius R, Held C, Berndt H (1997) Therapeutic efficacy of L-ornithine L-aspartate infusions in patients with cirrhosis and hepatic encephalopathy: results of a placebo-controlled, double-blind study. Hepatology 25: 1351–1360.
- Stauch S, Kircheis G, Adler G, Beckh K (1998) Oral L-ornithine L-aspartate therapy of chronic hepatic encephalopathy: results of a placebo-controlled, double-blind study. J Hepatol 28: 856–864.
- Chen M, Li R, Chen C, Gao X (2005) Observation of clinical effect of L-ornithine L-aspartate therapy on liver cirrhosis complicated by hepatic encephalopathy. Chin Libr Class 2588: 06-718-02.
- Schmid M, Peck-Radosavljevic M, König F, Mittermaier C (2010) A double-blind randomized, placebo-controlled trial of intravenous L-ornithine L-aspartate on postural control in patients with cirrhosis. Liver Int 30: 574–582.
- Mittal VV, Sharma BC, Sharma P, Sarin SK (2011) A randomized controlled trial comparing lactulose, probiotics, L-ornithine L-aspartate in treatment of minimal hepatic encephalopathy. Eur J Gastroenterol Hepatol 23: 725–732.
- Sidhu SS, Sharma BC, Goyal O, Kishore H (2018) L-Ornithine L-aspartate in bouts of hepatic encephalopathy. Hepatology 67: 700–710.
- Ahmad I, Khan AA, Alam A, Dilshad A, Butt AK, et al. (2008) L-ornithine-L-aspartate infusion efficacy in hepatic encephalopathy. J Coll Physicians Surg Pak 18: 684–687. [crossref]
- Sharma K, Pant S, Misra S, Dwivedi M (2014) Effect of rifaximin, probiotics and L-ornithine L-aspartate on minimal hepatic encephalopathy: a randomized controlled trial. Saudi J Gastroenterol 20: 225–232.
- Poo JL, Gongora J, Sanchez-Avila F, Aguilar-Castillo S (2006) Efficacy of oral L-ornithine L-aspartate in cirrhotic patients with hyperammonemic hepatic encephalopathy. Results of a randomized, lactulose-controlled study. Ann Hepatol 5: 281–288.
- Zhu GQ, Shi KQ, Huang S, Wang LR (2015) Systematic review with network meta-analysis: the comparative effectiveness and safety of interventions in patients with overt hepatic encephalopathy. Aliment Pharacol Ther 41: 624–635.
- Thumburu KK, Dhiman RK, Chopra M, Dutta U (2017) Comparative effectiveness of different pharmacological interventions for the treatment of minimal hepatic encephalopathy: a systematic review with network meta-analysis. J Clin Exp Hepatol 7: 6–7.
- Varakanahalli S, Sharma BC, Srivastava S, Sachdeva S (2018) Secondary prophylaxis of hepatic encephalopathy in cirrhosis of liver: a double-blind randomised controlled trial of L-ornithine L-aspartate versus placebo. Eur J Gastroenterol Hepatol.
- Higuera-de-la-Tijera F, Servin-Caamano AI, Salas-Gordillo F, Perez-Hernandez JL (2018) Primary prophylaxis to prevent the development of hepatic encephalopathy in cirrhotic patients with acute variceal bleeding. Can J Gastroenterol Hepatol 3015891.