DOI: 10.31038/JMG.2020333
Abstract
We suggested two important proteins (insulin-like growth factor-I (IGF-I) and ovocalyxin-32 (OCX-32) have crucial effects on muscle differentiation, growth, and reproductive traits in chicken production. On the association between IGF-I gene polymorphism and production traits, the evaluation of the associations between SNPs with reproductive traits suggests a positive effect of genotype AC with average egg weight at age of 30 (EW30) (P < 0.05) compared with genotype CC. We confirmed that the g.570 C > A polymorphism is significantly associated with average egg weight at age of 30 (EW30). On the other hand, for the association between OCX-32 gene polymorphism and production traits, two single nucleotide polymorphisms (SNPs) c.381G > C and c.494 A > C, were confirmed. Associations of OCX-32 genotypes with egg number (EN) were significant (p<0.05). From these findings we concluded that these markers should be considered for growth and production traits in chicken.
Keywords
Production traits, Chicken, IGF-I, OCX-32, PCR-RFLP
Introduction
Economically important livestock products are dependent on production and reproductive traits in domestic animals, which are under the control of multiple genes, mapping and analyzing polymorphisms of genes involved in the main metabolic pathways related to animal growth and distribution of nutrients to different tissues [1,2]. Understanding the genetic information of related genes is helpful for the selection and breeding course through marker assisted selection (MAS) in domestic animals. Candidate genes have well-known biological functions related to the development or physiology of important traits [3]. Such genes can encode structural proteins or a member in a regulatory or biochemical pathway affecting the expression of the trait [4] and can be tested as putative QTLs [5].
For poultry industry, meat and eggs are main products, for which some genes impact on such as insulin-like growth factor (IGF-I) and ovocalyxin-32 (OCX-32) have crucial roles on muscle differentiation, growth and reproduction. The presence of IGF-I in blood was detected as one of skeletal growth factors that produced in the liver tissue [6]. Therefore, insulin-like growth factor 1 is a key growth factor involved in a variety of biological processes [7,8]. Some study has shown that IGF-I mRNA levels were significantly lower in the low growth rate line than in the high growth rate line [9].
Recent studies on a single nucleotide polymorphism (SNP) in the chicken IGF-I gene have reported that there are significant associations between a polymorphism in this gene and its promoter in production and reproduction traits in poultry [10-12]. And the other hands, one group of detected eggshell matrix proteins related to major proteins of the egg white. Ovalbumin was the first egg white protein that explained in the matrix of the eggshell [13]. A second group contains proteins such as osteopontin that are also found in other tissues [14]. Finally, a third group of proteins includes those specific to the uterine tissue and to the eggshell that have only been detected in extracts of eggshell. Recent studies have shown associations between single nucleotide polymorphism of ovocalyxin-32 gene its family genes and egg production traits [15-18]. The eggshell contains some eggshell-specific matrix proteins such as ovocleidin-17, ovocleidin-116, ovocalyxin-32 and ovocalyxin-36 [14,19]. It was demonstrated that ovocalyxin-32 (OCX-32) is a 32 kDa protein that found in the outer region and cuticle of the shell [20]. Dunn et al. [16] reported that a single nucleotide polymorphism in the intron of the OCX-32 gene was associated with the thicknesses of the mammillary layer. Some studies have shown that low egg production strains expressed more transcripts of the OCX-32 gene in comparison of high strains at egg-laying stages and offered that the OCX-32 gene is a crucial marker that associated with egg production [21,22].
The objectives of the present study was 1) to detect SNPs of IGF-I and OCX-32 genes by developing PCR-RFLP methods, and 2) to investigate and analyze associations between those SNPs and growth and egg production traits in Mazandaran indigenous chicken.
Material and Methods
Growth and Egg Production Traits
The evaluated traits and their descriptions were presented in Table 1.
Experimental Population and Sampling
Chickens were raised in Native Chicken Breeding Station of Mazandaran, and they belonged to generation 19 of the breeding station pedigreed animals. Blood sample (1 mL) was taken in an EDTA-containing tube and all samples were freeze. Whole DNA was extracted by using DNA Extraction Kit [23]. The DNA samples were stored at -20ºC for use.
Primer Synthesis and PCR–RFLP Reactions
IGF-I analysis: Promoter region of the IGF-I gene amplified to a product of 361 bp using the oligonucleotide design tool Primer 5.0 software based on the IGF-I gene sequence of the fowl (Accession number: M74176). Primers were F: 5′-CTCTGCCACGAATGAAATGTGC-3′ and R: 5′-GGGAGCATTTGCCTTCTCTC-3′ for IGF-I gene. PCR method was used to optimize the reaction accuracy: 94ºC for 2 min, 30 cycles of 98ºC for 30 s, annealing at 55ºC for 30 s, 68ºC for 40 s, and a final extension at 72ºC for 7 min. Finally, PCR products were electrophoretically separated on 2% agarose gel (5 V/cm) and stained with ethidium bromide.
The fragment was amplified for PCR–restriction fragment length polymorphism (RFLP) analysis. An amplified fragment was digested by HinfI for detecting the g.570C > A genotypes. The restriction enzyme digestion was incubated at 37°C for 4 h.
OCX-32 Analysis
With forward primer 5′-CTCCAAACGTATGCTTCACTTA-3′ and reverse primer 5′-ATTCTTGTGTTCGGTTACTTGT-3′, approximately 342 bp covering complete exon-3 was obtained. As well as forward primer 5′-TGTTTCTGATGAAGAGCCAGA-3′ and reverse primer 5′-CTTTGCCACTCTGTAGGCTGT-3′, approximately 250 bp covering exon-4 was obtained. Two fragments containing the OCX-32 polymorphisms (NM_204534: c.381G>C; and NM_204534: c.494A>C) were amplified for PCR-RFLP analysis. An amplified fragment was digested with NcoI for detecting the c.381G>C and c.494A>C genotypes, respectively. The restriction enzyme digestions were incubated at 37°C for 4 h.
Statistical Analyses
The relationship between genes polymorphism and related traits were calculated using general linear model of SAS 9.0 (SAS Inc., Cary, NC, USA). The used models in matrix notation were as follows:
Y = Xb + Za + e
Where, Y is the vector of observations; b the vector of fixed effects of generation, sex and hatch; a the vector of random direct genetic effects; e the vector of random residual effects; X and Z are incidence matrices relating the observations to the respective fixed and direct genetic effects.
Results
The Production Traits
The means and SD of the traits measured in the chickens are shown in Table 1.
Table 1: Statistical description of data set for growth and egg production traits.
|
Traits |
No. of animal | Mean |
Coefficient of variation |
|
BW1 (gr) |
34,277 | 34.53 |
8.13 |
|
BW8 (gr) |
42,057 | 553.7 |
17.12 |
|
BW12 (gr) |
37,207 | 943.9 |
14.51 |
|
WSM (gr) |
30,137 | 1684 |
11.92 |
|
ASM (day) |
30,339 | 155.5 |
9.25 |
|
EN (number) |
30,349 | 36.66 |
39.76 |
|
EW1 (gr) |
26,284 | 40.21 |
15.77 |
|
EW28 (gr) |
16,215 | 45.91 |
8.43 |
|
EW30 (gr) |
18,021 | 47.12 |
8.52 |
|
EW32 (gr) |
17,945 | 48.22 |
8.31 |
|
EW12 (gr) |
17,837 | 48.62 |
9.34 |
|
AV (gr) |
27,715 | 45.84 |
13.34 |
|
EM (gr) |
27,725 | 1758 |
39.13 |
|
EINT (%) |
30,339 | 53.07 |
33.33 |
BW1, BW8, BW12 = Body Weight at Birth, 8, 12 weeks of age, WSM = Body Weight at sexual maturity, ASM = Age at first egg, EN = Egg Number, EW1 = Weight of First Egg, EW28, EW30 and EW32 = Average Egg Weight at 28, 30 and 32weeks of age respectively, EW12 = Average egg weight for First 12 weeks of production, AV = Average for EW28, 30 and 32, EM = Egg Mass (=EN×EW12), EINT = Egg Production Intensity (=Egg Number/Days Recording)×100).
Phenotypic Analysis and Sequence Analysis
IGF-I
The single nucleotide polymorphisms was located in the promoter region and produced an A→C substitution at base 570 (accession number M74176), which was the same mutation identified in previous studies [10,11,24]. Genotypes of chickens were investigated by PCR-RFLP (Figure 1). Genotype and allele frequencies of the SNP in chickens are shown in Table 2. In this population, the AA genotype had the highest frequency (0.62), followed by AC (0.32) and CC (0.06), and the A and C allele frequencies were 0.78 and 0.22, respectively.
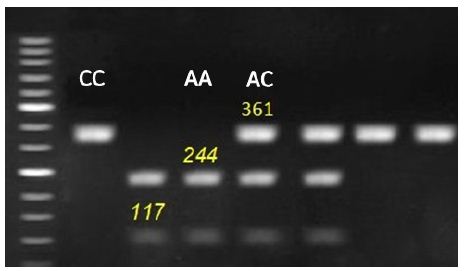
Figure 1: Representative genotyping of IGF-1 gene at locus g.570C > A. by agarous gel electrophoresis. Strands with 361 for CC genotype, 117, 244 and 361 for AC genotype and 117 and 244 for AA genotype appeared at this locus.
Table 2: Genotypic and gene frequency of IGF-I gene.
|
IGF-I |
||||
|
Frequency |
Allele | Frequency | No. |
Genotype |
|
0.78 |
A | 0.62 | 113 |
AA |
|
0.22 |
C | 0.32 | 59 |
AC |
| 0.06 | 11 |
CC |
||
| 1 | 183 |
Total |
||
OCX-32
The SNPs in exon 3 and 4 were a C→G substitution at base 381 (c.381 G > C; accession number NM_204534) in exon 3, and C→A substitution at base 494 (accession number NM_204534) in exon 4, respectively, which was the same mutation reported in the previous study [21].
We observed 3 band on gel for OCX-32 exon 3 (Figure 2). Three genotypes in this segment GG, GC and CC had the genotypic frequencies of 0.61, 0.26 and 0.13, respectively (Table 3). Two band patterns for OCX-32 exon-4 (Figure 3) were observed. Three genotypes in this segment AA, AC and CC had the genotypic frequencies of 0.11, 0.36 and 0.53, respectively (Table 3).
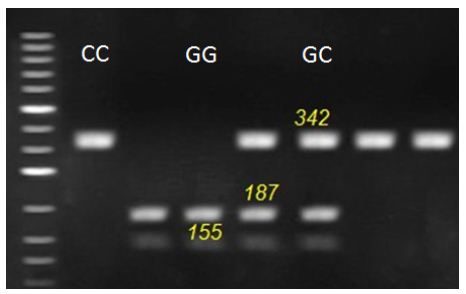
Figure 2: Representative genotyping of OCX-32 gene at locus c.381 G>C. by agarous gel electrophoresis. Strands with 342 for CC genotype, 155, 187 and 342 for GC genotype and 155 and 187 for GG genotype appeared at this locus.
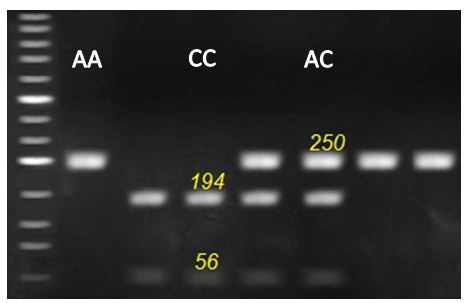
Figure 3: Representative genotyping of OCX-32 gene at locus c.494 A > C. by agarous gel electrophoresis. Strands with 250 for AA genotype, 56, 194 and 250 for AC genotype and 56 and 194 for CC genotype appeared at this locus.
Table 3: Genotypic and gene frequency of OCX-32 gene.
|
SNP |
Frequency genotype |
Frequency allele |
|||
|
c.381G>C |
GG | GC | CC | G |
C |
|
0.61 |
0.26 | 0.13 | 0.74 |
0.26 |
|
|
c.494A>C |
AA | AC | CC | A |
C |
|
0.11 |
0.36 | 0.53 | 0.29 |
0.71 |
|
Associations between Genotypes and Production Traits or Breeding Values
IGF-I
Results on the effects of IGF-I SNP on production and growth traits are shown in Table 4. The g.570 C > A genotype was significantly associated with average egg weight at age of 30 (EW30) (P < 0.05) in this population. No significant association was found between the IGF-I SNP and other traits (Table 4).
Table 4: Association of the IGF-I genotypes at the growth and egg production traits (Mean ± S.E.).
|
Traits |
|||
|
Genotype AA |
Genotype AC |
Genotype CC |
|
| WSM |
1726.18 ± 20.22 |
1740.25 ± 17.84 |
1765.15 ± 42.12 |
| ASM |
175.81 ± 1.06 |
177.97 ± 0.79 |
174.68 ± 2.79 |
| EN |
39.28 ± 0.89 |
40.07 ± 0.80 |
40.09 ± 1.70 |
| EW28 |
44.30 ± 0.52 |
45.51 ± 0.46 |
43.10 ± 1.01 |
| EW30 |
48.44 ± 0.49 ab |
48.41 ± 0.45 a |
46.27 ± 0.96 b |
| AV |
51.91 ± 0.44 |
51.38 ± 0.40 |
51.03 ± 0.83 |
| EM |
1929.45 ± 446.35 |
2019.28 ± 41.43 |
1958.00 ± 87.54 |
| EINT |
59.29 ± 2.64 |
61.26 ± 1.49 |
61.03 ± 3.07 |
a,bValues with different superscripts within the same row differ significantly (P<0.05).
OCX-32
Results on the effects of OCX-32 SNP on growth and production traits are shown in Table 5. At exon 3, genotype GC had higher egg number compared with genotype GG (P < 0.05, Table 5). At exon 4, genotype CC was significantly associated with egg number (P < 0.05, Table 5) compared with genotype AC.
Table 5: Effects of c.381G>C and c.494A>C SNPs of the OCX-32 gene on growth and production traits (least squares means ± SE).
|
SNP |
c.381G>C |
c.494A<C |
||||||
|
Genotype |
GG | GC | CC | AA | AC |
CC |
||
|
BW12 (gr) |
751.33 ± 14.36 | 733.25 ± 16.24 | 707.71 ± 29.69 | 688.82 ± 26.03 | 732.47 ± 14.62 |
756.56 ± 13.50 |
||
|
WSM (gr) |
1738.36 ± 21.54 | 1729.77 ± 26.07 | 1706.98 ± 53.00 | 1685.13 ± 46.32 | 1713.00 ± 24.07 |
1761.97 ± 23.05 |
||
|
ASM (day) |
175.37 ± 1.66 | 177.76 ± 1.10 | 179.32 ± 3.25 | 172.34 ± 1.83 | 173.24 ± 1.99 |
172.42 ± 1.90 |
||
|
EN (number) |
41.30 ± 0.79 b | 43.64 ± 0.96 a | 43.42 ± 1.95 ab | 41.83 ± 1.72ab | 43.54 ± 0.89 a |
341.39 ± 0.85 b |
||
|
EW28 (gr) |
44.56 ± 0.45 | 44.62 ± 0.57 | 45.51 ± 1.16 | 43.29 ± 0.98 | 44.53 ± 0.50 |
45.18 ± 0.50 |
||
|
EW30 (gr) |
48.25 ± 0.35 | 47.84 ± 0.44 | 49.31 ± 1.01 | 46.81 ± 0.82 | 48.65 ± 0.49 |
48.13 ± 0.49 |
||
|
EW32 (gr) |
52.56 ± 0.43 | 52.01 ± 0.52 | 49.70 ± 1.06 | 51.10 ± 0.97 | 52.06 ± 0.49 |
52.54 ± 0.47 |
||
|
EW84 (gr) |
51.68 ± 0.49 | 51.46 ± 0.60 | 49.71 ± 1.21 | 49.38 ± 1.10 | 50.31 ± 0.56 |
51.81 ± 0.53 |
||
|
AV (gr) |
50.22 ± 0.38 | 50.19 ± 0.46 | 48.19 ± 0.94 | 48.26 ± 0.83 | 50.03 ± 0.43 |
50.47 ± 0.41 |
||
|
EM (gr) |
1947.08 ± 40.96 | 2070.16 ± 49.21 | 1987.59 ± 100.22 | 1907.50 ± 88.20 | 2061.73 ± 45.84 |
1958.06 ± 44.32 |
||
a,bValues with different superscripts within the same row differ significantly (P<0.05).
Discussion
In the present study, the g.570 A < C polymorphism of the IGF-I promoter was detected in Mazandaran indigenous chicken: allele frequencies for A and C were 0.78 and 0.22, respectively. The g.570 A < C polymorphism has been reported as a candidate mutation influencing growth and carcass traits [10,11,25]. Previous studies have shown that the g.570 A > C polymorphism is significantly related to body weight at 107 days of age [10], 2, 4, 6 and 8 weeks of age [11], and 5 weeks of age [25].
For production traits, however, no significant differences were found in the association between the g.570 A < C polymorphism and other traits. In contrast, the g.570 A < C polymorphism is strongly assumed to be involved in 30 weeks (EW30). These results support the notion that selection of average egg weight at age of 30 weeks (EW30), is a powerful method to increase production traits and the g.570 A<C polymorphism might become a marker for elevating average egg weight at age of 30 (EW30). In particular, we propose that the identification of g.570 A < C genotypes may be useful in the selection for reproductive traits. To make further progress, it is necessary to investigate the associations between the g.570 A<C genotypes and production traits in the other breed.
The relationship between the IGF-I promoter mutation and growth or carcass traits has been studied in some breeds of chicken and cattle. For example, effects of the polymorphism IGF-I gene were surveyed on egg quality in Wenchang chicken [12]. Some results showed single nucleotide polymorphisms 512-bp upstream from the start codon had significant associations with weight gain during the first 20 days after weaning and on-test weight in Angus cattle [26]. Furthermore, the same report indicated that the g.570 C > A substitution A→C in the promoter region involved the suppression of one potential CdxA transcription factor binding site. Hence, the different alleles detected in the present study might alter the transcription rate and the gene expression level of IGF-I, thereby affecting circulating IGF-I concentrations and muscle development.
An association between the single nucleotide polymorphisms of the OCX-32 gene and the thicknesses of the mammillary layer was recently reported by Dunn et al. [16]. In the present study, the SNP of the OCX-32 gene were significantly associated with body weight at age of 12 weeks, average of EW28, EW30 and EW32, egg number and average egg weight at age of 32 and 84 weeks. We did not find any significant association between these single nucleotide polymorphisms and the other investigated traits. In the case of c.494 A > C SNP in exon 4 implied that the chicken OCX-32 gene may affect reproductive and production traits related traits simultaneously. Yang et al. [22] showed that the OCX-32 expression levels and EPR were related. Therefore, to confirm the causal mutation derived by only a single SNP, we need to evaluate the effects in different breeds and lines of chickens and investigate the function of this gene in detail. Our results show that detection and utilization of candidate gene mutations and DNA markers obtained by whole-genome scanning may directly improve growth, production traits and other economic traits within the same breeds. In particular, we propose that the identification of genotypes may be useful in the selection for production and reproduction traits. To make further progress, it is necessary to investigate the associations between the genotypes and traits in the other breeds. This result shows that OCX-32 gene can be used as a candidate marker in marker-assisted selection. Further functional analysis is essential to ascertain the effects of the OCX-32 exon polymorphism.
Abbreviations
IGF-I: Insulin-like growth factor-1
OCX-32: Ovocalyxin-32
BW1, BW8, BW12: Body Weight at Birth, 8, 12 weeks of age
WSM: Body Weight at sexual maturity
ASM: Age at first egg
EN: Egg Number
EW1: Weight of First Egg
EW28, EW30 and EW32: Average Egg Weight at 28, 30 and 32weeks of age respectively
EW12: Average egg weight for First 12 weeks of production
AV: Average for EW28, 30 and 32
EM: Egg Mass
EINT: Egg Production Intensity
Acknowledgement
The authors are thankful to the researcher, F. Marandi for positive feedback on this research project.
References
- Schwerin M, Brockmann G, Vanselow J, Seyfert HM (1995) Perspectives of molecular genome analysis in livestock improvement-an overview. Animal Research Development 42: 14-26.
- Bahrami A, Miraei-Ashtiani SR, Mehrabani-Yeganeh H, Banani-Rad H, Behzadi SH (2014) The association between polymorphism of the GH1 gene and changes in protein structure and carcass traits in Mehraban sheep (Ovis aries). Anim Prod Sci 55: 661-665.
- Rothschild MF, Soller M (1997) Candidate gene analysis to detect genes controlling traits of economic importance in domestic livestock. Probe 8: 13-20.
- Bryne PF, Mcmullen MD (1996) Defining genes for agricultural traits: QTL analyses and the candidate gene approach. Probe 7: 24-27.
- Yao J, Aggrey SE, Zadworny D, Hayes JF, Kühnlein U (1996) Sequence variations in the bovine growth hormone gene characterized by Single-Strand Conformation Polymorphism (SSCP) analyses and their association with milk production traits in Holstein. Genetics 144: 1809-1816. [crossref]
- Daughaday WH, Salmon WD (1999) The origins and development of the somatomedin hypothesis. In: Contemporary Endocrinology: The IGF System, Rosenfeld, R. Roberts (Eds.). Humana Press Inc Totowa NJ 1-15.
- Adam CL, Gadd TS, Findlay PA, Wathes DC (2000) IGF-I stimulation of luteinizing hormone secretion, IGF-binding proteins (igfbps) and expression of mrnas for igfs, IGF receptors and igfbps in the bovine pituitary gland. Journal of Endocrinology 166: 247-254. [crossref]
- Shen WH, Wisniowski P, Ahmed L, Boyle DW, Denne SC, et al. (2003) Protein anabolic effects of insulin and IGF-I in the ovine fetus. American Journal of Physiology and Endocrinology Metabolism 284: 748-756. [crossref]
- Beccavin C, Chevalier B, Cogburn LA, Simon J, Duclos MJ (2001) Insulin-like growth factors and body growth in chickens divergently selected for high or low growth rate. Journal of Endocrinology 168:297-306. [crossref]
- Amills M, Jimenez N, Villalba D, Tor M, Molina E (2003) Identification of three single nucleotide polymorphisms in the chicken insulinlike growth factor 1 and 2 genes and their associations with growth and feeding traits. Poultry Science 82: 1485-1493. [crossref]
- Zhou H, Mitchell AD, Mcmurtry JP, Ashwell CM, Lamont SJ (2005) Insulin-like growth factor-I gene polymorphism associations with growth, body composition, skeleton integrity, and metabolic traits in chickens. Poultry Science 84: 212-219. [crossref]
- Lei M, Luo C, Peng X, Fang M, Nie Q, et al. (2007) Polymorphism of growth-correlated genes associated with fatness and muscle fiber traits in chickens. Poultry Science 86: 835-842. [crossref]
- Hincke MT, Tsang CPW, Courtney M, Hill V, Narbaitz R (1995) Purification and characterization of a soluble matrix protein of the chicken eggshell (ovocleidin 17). Calcif Tissue Int 56: 578-583. [crossref]
- Pines M, Knopov V, Bar A (1995) Involvement of osteopontin in egg shell formation in the laying chicken. Matrix Biology 14: 765-771.
- Dunn IC, Miao YW, Morris A, Romanov MN, Wilson PW, et al. (2004) A study of association between genetic markers in candidate genes and reproductive traits in one generation of a commercial broiler breeder hen population. Heredity 92: 128-134. [crossref]
- Dunn IC, Joseph NT, Bain M, Edmond A, Wilson PW, et al. (2008) Polymorphisms in eggshell organic matrix genes are associated with eggshell quality measurements in pedigree Rhode Island Red hens. Animal Genetics 40: 110-114. [crossref]
- Honkatukia M, Reese K, Preisinger R, Tuiskula-Haavisto M, Weigend S, et al. (2005) Fishy taint in chicken eggs is associated with a substitution within a conserved motif of the FMO3 gene. Genomics 86: 225-232. [crossref]
- Cui JX, Du HL, Liang Y, Deng XM, Li N, et al. (2006) Association of polymorphisms in the promoter region of chicken prolactin with egg production. Poultry Science 85: 26-31. [crossref]
- Gautron J, Hincke MT, Dominguez-Vera JM, Garcia-Ruiz JM, Nys Y (2001) Ovotransferrin is a matrix protein of the hen eggshell membranes and basal calcified layer. Conn Tissue Research 42: 255-267. [crossref]
- Gautron J, Murayama E, Hincke MT, Nys Y (2005) Chicken eggshell matrix proteins related to anti-bacterial protein families. Proceedings of the 11th European Symposium on the Quality of Eggs and Egg Products, Doorwerth, Netherlands.
- Uemoto Y, Suzuki C, Sato S, Ohtake T, Sasaki O, et al. (2009) Polymorphism of the ovocalyxin-32 gene and its association with egg production traits in the chicken. Poultry Science 88: 2512-2517. [crossref]
- Yang KT, Lin CY, Liou JS, Fan YH, Chiou SH (2007) Differentially expressed transcripts in shell glands from low and high egg production strains of chickens using cdna microarrays. Animal Reproduction Science 101: 113-124.
- Miller SA, Dykes DD, Polesky HF (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucloic Acids Research 16: 1215. [crossref]
- Shinichi S, Tsuyoshi O, Yoshinobu U, Yumi O, Eiji K (2012) Polymorphism of insulin-like growth factor 1 gene is associated with breast muscle yields in chickens. Animal science journal 83: 1-6. [crossref]
- Bennett AK, Hester PY, Spurlock DE (2006) Polymorphisms in Vitamin D receptor, osteopontin, insulin-like growth factor 1 and insulin, and their associations with bone, egg and growth traits in a layer – broiler cross in chickens. Animal Genetics 37: 283-286. [crossref]
- Mann K, Gautron J, Nys Y, Mckee MD, Bajari T, et al. (2003) Disulfide-linked heterodimeric clusterin is a component of the chicken eggshell matrix and egg white. Matrix Biology 22: 397-407. [crossref]