Abstract
Rivastigmine, used in the treatment of Alzheimer’s Disease, is in liquid state at controlled room temperatures. This project was aimed at developing a 0.5-mL isotonic liquid containing rivastigmine with the choice of 0.9% NaCl or 5% Dextrose Solutions for Injection as a simulating study to formulate a liquid dosage form per description of 3M hollow microneedles (https://multimedia.3m.com/mws/media/1004089O/solid-microstructured-transdermal-system-smts-sell-sheet.pdf). The surfactants were compared among Span 20, Span 80, Tween 40 and Tween 80 with benzyl alcohol or chlorobutanol as a preservative. Those formulations which formed microemulsions were further studied for stability at 4°C and 25°C up to one month. HPLC confirmed that there were no drug losses among the four microemulsions. Based on zeta potential and particle size analysis, Tween 80 with benzyl alcohol in 0.9 % NaCl is the best project formulation.
Keywords
Benzyl alcohol, chlorobutanol, Hollow Microstructured Transdermal System (hMTS), microneedles, rivastigmine, Tween and Span
Introduction
Alzheimer’s disease is an irreversible, progressive neurodegenerative disorder subsequently becoming a common cause of death [1]. Alzheimer’s is the most common cause of dementia affecting an estimated 5.8 million people in the United States [1]. In 2018, the approximate cost of caring for people with Alzheimer’s disease and other dementias was $290 billion USD, making it a huge economic burden for both patients’ families and our society and leading to a major public health problem. It is a huge economic burden for patients’ families and our society. Unfortunately, there has not been an effective treatment for Alzheimer’s disease thus far. One of the reasons is that the exact mechanism of disease development is still unclear. Acetylcholinesterase inhibitors such as donepezil, rivastigmine and galantamine, and N-methyl D-asparate receptor antagonist, and memantine are therapeutic agents. Among them, rivastigmine has the advantage of inhibiting both acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE). It is also superior to the aforementioned other drugs in having two US FDA approved commercial dosage forms, that is oral capsules and transdermal patches (Table 1) [2,3]. Unfortunately, nausea and vomiting have been reported by patients taking rivastigmine oral dosage form [4]. Transdermal delivery system has a better tolerability and more efficacy compared to oral capsules [5] and enables patients who have difficulties in swallowing to take medicine more easily, and less frequently (once daily, Table 1b) [6]. However, it was reported that transdermal patch may cause skin irritation when a 24-h patch is worn. Therefore, this study focuses on the feasibility of formulating rivastigmine for use of microneedle administration and formulation characterizations. Microneedle has been gathering attention on their merits, including shorter time to reach Cmax and penetrating more drugs, especially macromolecules, than the transdermal patch dosage form [7].
Table 1: Rivastigmine dosage forms on the market: (a) oral capsules, and (b) extended release transdermal films [2,3].
(a) Oral capsules
| Strength | Manufacturers | Applicant Holder |
| EQ 1.5, 3, 4.5, 6 mg BASE | Brand name product | Novartis |
| EQ 1.5, 3, 4.5, 6 mg BASE | Generic products | Alembic; Apotex; Aurobindo; Cadila; Chartwell; Dr. Reddy’s; Macleods; Orchid; Sun; Watson |
(b) Extended release transdermal film
| Strength | Manufacturers | Applicant Holder |
| 4.6, 9.5, 13.3 mg/24 h | Brand name product | Novartis |
| 4.6, 9.5, 13.3 mg/24 h | Generic products | Alvogen malta operations;
Amneal; Mylan; Zydus |
Microneedles devise a pain-free penetration feature (minimally invasive device which pierces through the stratum corneum without touching the nerve endings and capillaries). Its other merits are avoidance of the first-pass, improvement of skin permeability and permeation, the delivery of both small and large molecules, achieve stable plasma concentrations for up to 7 days and possibly improvement of bioavailability. There are four subtypes of microneedles: solid, coated, dissolving, and hollow [8-10].
Colloidal Dispersions, Emulsions and Microemulsions
Colloids are heterogeneous mixture systems. Colloidal dispersion is characterized by their particle sizes and shapes. A particle size ranged between 1 nm and 1 μm makes the properties of colloids fall between solution and suspensions. Whether their particles are small enough to separate on standing or are large enough to scatter light (a phenomenon called Tyndall effect, which makes the liquid’s appearance cloudy or opaque) depends on the particle size. Therefore, colloidal dispersions are further divided into molecular colloids (solutions), association colloids and dispersion colloids [11]. Emulsions are composed of two or more immiscible liquids and suitable emulsifying agent(s), which appear milky and nontransparent because of the different optical refraction of the components. Depending on the hydrophilic and lipophilic characters, emulsions may be divided into oil in water (O/W) and water in oil (W/O) subtypes. In addition to these, multiple phases of emulsions exist as W/O/W and O/W/O microemulsions. Emulsions are kinetically stable but thermodynamically unstable based on their dispersed state and the corresponding high interfacial energy. On the other hand, microemulsions are fundamentally different from emulsions in terms of appearance, structure, and properties, which are considered between micellar solution and emulsions. Their appearances vary from transparent to opalescent, moderately viscous, and optically isotropic. Microemulsions are thermodynamically stable [11,12].
Materials
Rivastigmine (1 g and 0.5 g HY-17368 in two separate orders; liquid state in controlled room temperature) was obtained from MedChem Express (Monmouth Junction, NJ). 5 % Dextrose (also known as D5W, Lot J4J577) and 20 mL syringes were acquired from Cardinal Health (Dublin, OH). Sodium chloride (Lot: 284929), Tween 40 (polyxyethylene sorbitan monopalmitate, Lot: D5ZONHK, TGI Tokyo, Japan), methanol and 0.2 micron VWR syringe filters were ordered from VWR International (Randor, PA). Span 20 (Sorbitan monolaurate, Lot: C1885020), Span 80 (sorbitan monooleate, Lot: C182545), Tween 80 (polysorbate, Lot: C188508), benzyl alcohol (Lot: C171703), chlorobutanol, and sodium phosphate dibasic were bought from PCCA (Houston, TX).
Methods
Determination of High Performance Liquid Chromatographic (HPLC) Column
USP43-NF38 2020 recommended to assay rivastigmine and its tartrate salt by using Symmetry C18, Nova Pak C18, and Spherisorb C8 columns [13]. Buffer was first prepared as 8.9 g/L of dibasic sodium phosphate dihydrate in water (0.05 M). Mobile phase was the mixture of methanol and Buffer (0.05M sodium phosphate dibasic) in 58:42 v/v ratio. The solution was let cool to controlled room temperature before the pH adjustment to the value of 8.45 using phosphoric acid. The LC assay conditions were flow rate of 1.0 mL/min, and column temperature at 40°C. The detection wavelength was chosen at 214 nm for all study LC columns with run time at 20 minutes initially. When no impurities were seen, the run time was reduced to 15 minutes or shorter to avoid the generation of biohazard wastes from the prolonged use of mobile phase containing methanol.
Standard Curve
Ten mL of rivastigmine (which density is 1.0 g/mL) initially placed in a volumetric flask to determine its weight. A sufficient amount of the mobile phase was then added to make it into 10 mL as the stock solution, which has a concentration of 1 mg/mL (because the density of rivastigmine is 1 g/mL). This stock solution was further diluted 5-fold with mobile phase each time for sequentially six times with the eighth sample only being diluted two-fold from the seventh sample. One mL of each was transferred into a HPLC vial for assay injection. Accuracy is determined based on how close a measured value is to the actual (true) value. One of the actual (true value) may rely on the use of USP Reference Standard for comparison. Precision is determined based on how close the measured values are to each other. Since Rivastigmine USP Reference Standard was not available for the free base form to report Accuracy (but available as Rivastigmine Tartrate). This project reported the Precision instead. The HPLC conditions were as the follows: wavelength at 214 nm; flow rate 1.0 mL/min; run time 12 min; column temperature 40°C.
Formulation Preparation
3M Hollow Microstructured Transdermal System (hMTS) is an integrated device containing actuator, glass injection cartridge, delivery spring, adhesive, hollow microstructured array and application spring with information available at 3M, St. Paul, MN [12]. Each glass cartridge may house 0.5 mL to 2 mL of intradermal delivery solution (Figure 1a and 1b). In this project, 4.6 μL of rivastigmine (that is 4.6 mg) was selected to develop into 0.5 mL of liquid dosage form as a single dose. As aforementioned, rivastigmine is in liquid state at controlled room temperature, its density is 1.0 g/mL. Therefore, rivastigmine was measured by volume, instead of weight in this project. Suitable excipients such as solution for injection, surfactant and preservative were added into the final volume of 0.5046 mL, which is within the glass cartridge capacity between 0.5 mL to 2 mL. The selection of excipients such as Solutions for Injection, surfactants, and preservatives are briefly described as follows. Isotonic 0.9% saline and 5% dextrose (D5W) were chosen as Solutions for Injection. Since rivastigmine is lipophilic and in liquid state at controlled room temperature (23 ± 2°C), four surfactants (Span 20; Span 80; Tween 40 and Tween 80 (Figure 2) were added respectively to check compatibility. In addition, two preservatives (benzyl alcohol or chlorobutanol) were included. Each formulation in the saline group was composed of 0.9 g of NaCl, 5.0 g of surfactants and 1.0 g of benzyl alcohol (or 0.5g of chlorobutanol) in 100 mL final volume. The D5W group contained 5.36 g of surfactants, 1.0 g of benzyl alcohol (or 0.5 g of chlorobutanol) with sufficient amount of D5W in the total of 100 mL final volume. After mixing, the degree of transparency vs. cloudiness of all formulations were visually observed to determine candidacy for further testing. Rivastigmine 0.92 mL was taken and mixed with each 100 mL of liquid to assess particle size, zeta potential and conduct HPLC.
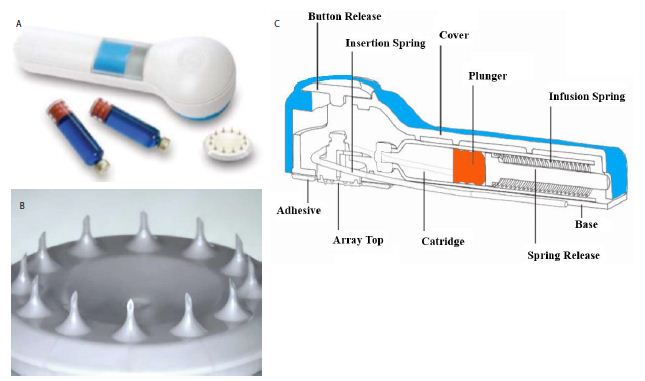
Figure 1: Hollow Microneedle Transdermal System (hMTS): (a) 3M device, (b) scheme showed the inside view of the integrated device, and (c) polymer microneedle array with 12 hollow microneedles, each approximately 1500 µm [12].

Figure 2: The appearance of surfactants from left to right: Span 20 (HLB 8.6), Span 80 (HLB 4.0), Tween 40 (HLB 15.6) and Tween 80 (HLB 15.0).
Visual Characterization
The miscibilities of resultant formulations after rivastigmine mixed with one of the two Solutions for Injection, one of the four surfactants and one of the two preservatives were visually observed.
Particle Size Analysis and Zeta Potential
Rivastigmine (9.2 μL, density 1 g/mL) was added to the aforementioned different blank formulations into the total volume of 2 mL for each. The formulations were analyzed by the NanoBrook 90 Plus Particle Sizer. The refractive indexes of rivastigmine, Span 20, Tween 40, Tween 80, benzyl alcohol, and chlorobutanol used in particle size analysis were 1.518, 1.474, 1.470, 1.473, 1.539, and 1.491, respectively (Refractive index of a medium is the ratio of the speed of light in vacuum to the speed of light in the medium. Therefore, it has no units). The zeta potentials of formulations were analyzed using NanoBrook 90 Plus Zeta Potential Reader.
One-Month Stability Study
Each formulation sample containing 4.6 μL of rivastigmine (equivalent to 4.6 mg) was subject to HPLC assay as Time 0 samples. The LC method was adopted from USP-NF 2020 [13]. One half mL of this liquid plus 4.6 μL (4.6 mg) of rivastigmine was then taken into an amber vial and crimped with an aluminum cap (as a single dose) and stored at 4°C and 25°C respectively (n = 3) for one month prior to HPLC assay to determine the drug loss and compare the stabilities among formulation candidates.
Statistics
AUCs obtained from HPLC were statistically assessed by one way or two way ANOVA tests when normality and equal variance were met. Kruska-Wallis test was used if normality and equal variance were not met. Tukey as postdoc was used to compare the three groups. Population differences are considered significant at P < 0.05.
Results
Visual Observation
When using D5W as the Solution for Injection and mixed with one of the four surfactants, the Span 20 group looked homogeneous, but Span 80 appeared as heterogeneous. Tween 40 formed a yellowish clear solution. Tween 80 was a pale yellowish clear solution. Therefore, Span 80 was excluded from further experiments. Next, one of the two preservatives was added into these formulations. Preservative (either benzyl alcohol and chlorobutanol) in Span 20 formed milky microemulsion (Table 2). Benzyl alcohol with Tween 40 resulted in colloidal dispersion, while chlorobutanol formed microemulsion. Like Tween 40, chlorobutanol with Tween 80 formed microemulsion but benzyl alcohol had colloidal dispersion. Using 0.9 % NaCl for Solution for Injection, in contrast with D5W, Span 20 and Span 80 were heterogeneous and immiscible. From these results, they were eliminated from the formulation. Tween 40 in 0.9% NaCl was a yellowish clear solution. When benzyl alcohol was added as a preservative, it formed yellowish colloidal dispersion, while chlorobutanol formed precipitations. Tween 80 in 0.9% saline was also a clear solution. When either preservative was added to Tween 80, they formed clear microemulsion.
Table 2: Miscibilities of surfactants and preservatives in D5W or 0.9% NaCl Solution.
|
Surfactant |
Preservative |
D5W |
0.9% NaCl |
| Span 20 | Benzyl Alcohol |
Emulsion |
Non-miscible |
| Chlorobutanol |
Emulsion |
Non-miscible |
|
| Span 80 |
Non-miscible |
Non-miscible |
|
| Tween 40 | Benzyl Alcohol |
Colloidal dispersion |
Colloidal dispersion |
| Chlorobutanol |
Microemulsion |
Precipitation |
|
| Tween 80 | Benzyl Alcohol |
Colloidal dispersion |
Microemulsion |
| Chlorobutanol |
Microemulsion |
Microemulsion |
Particle Size and Zeta Potential Analyses
Span 20 with each preservative in D5W, Tween 80 with chlorobutanol in D5W and Tween 80 with each preservative in 0.9% NaCl were good candidates only in terms of particle sizes (Table 3). Among D5W, Tween 40 was significant different with Span 20 and Tween 80. However, there was no significant difference by using one or the other preservatives. Within benzyl alcohol in D5W, Span 20 and Tween 40 showed significantly different. Nevertheless, Span 20 and Tween 80 or Tween 40 and Tween 80 showed no difference. Furthermore, within chlorobutanol, between Tween 40 and Span 20 or Tween 80 showed a significant difference. Among the sample groups made of 0.9 % NaCl as Solution for Injection, there is no significant difference neither caused by surfactants nor by preservatives. Tween 80 with chlorobutanol in D5W and Tween 80 with either preservative in 0.9% NaCl can especially be considered as good candidates at time zero. Zeta potentials were also showed in the first right column of Table 3. There was no significant difference in terms of which surfactant or preservative was used in D5W or 0.9% NaCl. This suggests the stabilities of all formulation were similar at time zero.
Table 3: Particle sizes and Zeta potentials of six formulas using D5W as Solution for Injection and three formulas using 0.9% NaCl as Solution for Injection.
(a) D5W as Solution for Injection
| Surfactant |
Preservative |
Particle Size (nm)
Mean ± SD |
Zeta Potential (mV) |
| Rivastigmine in D5W alone |
434.27 ± 74.90 |
– | |
| Span 20
|
Benzyl Alcohol |
105.08 ± 25.53 |
-2.40 ± 5.85 |
|
Chlorobutanol |
148.67 ± 60.42 |
-4.49 ± 9.67 |
|
| Tween 40 |
Benzyl Alcohol |
6356.20 ± 6314.59 |
-4.90 ± 1.26 |
|
Chlorobutanol |
7186.41 ± 5069.88 |
-2.14 ± 3.10 |
|
| Tween 80 |
Benzyl Alcohol |
1749.11± 1902.47 |
-5.88 ± 0.62 |
|
Chlorobutanol |
10.33 ± 0.17 |
-1.31 ± 4.77 |
|
(b) NaCl as Solution for Injection
| Surfactant |
Preservative |
Particle Size (nm)
Mean ± SD |
Zeta Potential (mV) |
| Rivastigmine in 0.9% NaCl alone 360.93 ± 16.68 | – | ||
| Tween 40 |
Benzyl Alcohol |
13063.80 ± 13634.23 |
-4.13 ± 6.75 |
| Tween 80 |
Benzyl Alcohol |
12.74 ± 0.17 |
10.54 ± 16.86 |
|
Chlorobutanol |
11.49 ± 0.10 |
5.46 18.06 |
|
HPLC Assay
Column Selections and Standard Curve Linearity Range
Three LC columns (Symmetry C18, Nova Pak C18, and Spherisorb C8) were evaluated in this project. The tailing factors of column kinetics showed that Symmetry C18 was the best column to assay both rivastigmine and its tartrate salt form. The limit of detection of rivastigmine dissolved in mobile phase, and assayed by HPLC according to the method described in Section 5.5 was 0.00032 μL (0.0000320 mg/mL). The limit of quantification was 0.00064 μL (0.0000640 mg/mL). The linearity ranged from 0.000032 mg/mL to 1.0 mg/mL.
The AUC of the formulations which were compounded with different combinations of Solutions for Injection, surfactants and preservatives were assayed and converted into concentrations by applying with an established standard curve. In the group of using D5W as the Solution for Injection, whether the preservative was benzyl alcohol or chlorobutanol, the sample containing Span 20 had a significantly low concentration than those containing Tween 40 and Tween 80 (n = 4, p < 0.01, Figure 3a). There was no difference between either preservative (benzyl alcohol and chlorobutantol) whether the surfactant was Span 80, Tween 40 or Tween 80 (Figure 3a). When 0.9% NaCl solution was used as the solution for injection, there were no differences between Tween 40 and Tween 80 as surfactant while benzyl alcohol was the preservative (Figure 3b). Also, when Tween 80 was used as the surfactant, there was also no difference in using either preservative. The formulation containing benzyl alcohol and Tween 40 was not different to the formulation containing chlorobutanol and Tween 80 in 0.9% NaCl (n = 3, p > 0.05, Figure 3b).
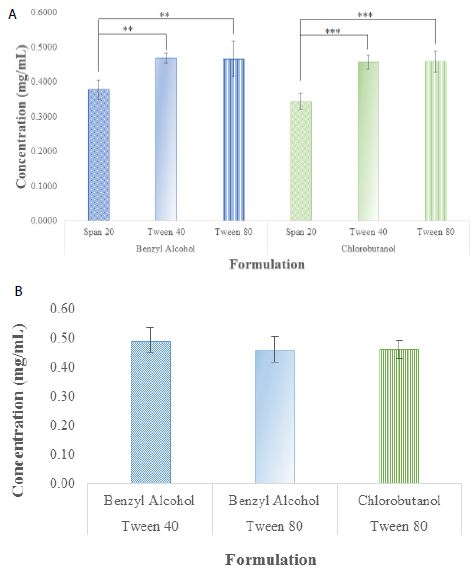
Figure 3: The rivastigmine concentrations of time 0 formulation candidates after chromatographic AUC being converted into concentrations using standard curves: (a) they were differed when D5W was used as the Solution for Injection; while (b) there was no differences when 0.9% NaCl solution was used as the solution for injection. (**p < 0.01, and ***p < 0.001).
One-Month Stability Study
Table 2 showed four of the studied formulations formed into microemulsion. They were further followed up with a stability study at 4°C and 25°C. They were Tween 40 and Tween 80 with chlorobutanol in D5W, and Tween 80 with either chlorobutanol or benzyl alcohol in 0.9% NaCl. When the samples were assayed by HPLC for drug content, there was no significant difference between time 0 and one-month in the metal-cap sealed glass vial samples stored at 4°C and 25°C, respectively (Table 4).
Table 4: The Concentration of Each Formulation at Time 0 and Stored at 4 and 25°C.
| Solution for Injection |
Surfactant |
Preservative | Mean ± SD (n = 3) | ||
| Time 0 | 4°C |
25°C |
|||
| D5W |
Tween 40 |
Chlorobutanol | 0.5165 ± 0.06047 | 0.5178 ± 0.06480 |
0.5537 ± 0.06496 |
| D5W |
Tween 80 |
Chlorobutanol | 0.4655 ± 0.03859 | 0.4571 ± 0.04455 |
0.4693 ± 0.03319 |
| 0.9 % NaCl |
Tween 80 |
Benzyl Alcohol | 0.4358 ± 0.01938 | 0.4472 ± 0.00303 |
0.4424 ± 0.01940 |
| 0.9 % NaCl |
Tween 80 |
Chlorobutanol | 0.4519 ± 0.01719 | 0.4490 ± 0.00949 |
0.4539 ± 0.01468 |
After One-Month
Discussion
The stability test performed by EMEA was for up to 5 years reported that rivastigmine free base is very sensitive to oxidation, moisture and heat [14]. Degradation is accelerated by the influence of heat. Therefore, it is recommended to be stored at 5 ± 3°C with protection from light and with protective gas [14]. Although the commercial Exelon Rivastigmine Patches also used free base drug, and the FDA approved labels indicated that they may be stored at controlled room temperature, perhaps it is because this commerical patch is packaged in aluminum pouch with an internal polymer coat and external composite printable surface. In our study, we compared the product concentrations at Time 0 and after one month and found no drug loss threatened by oxidation and moisture whether the formulations were stored at 4°C or 25°C. It was because our formulation was packed in metal-cap sealed glass vials prior to being subject to each storage temperature. Since the product of this project is in liquid form (unlike the transdermal patches and oral capsules which are solids), it is suggested that the long-term storage temperature be studied.
Conclusion
When the rivastigmine concentrations among the three surfactants (Span 20, Tween 40 and Tween 80) using benzyl alcohol as the preservative in D5W were compared, Span 20 was significantly different from Tween 40 (p < 0.01). It was also different from Tween 80 (p < 0.01), while Tween 40 and Tween 80 were no different (p = 0.981). This identified that Span 20 is not a suitable surfactant. The same results were acquired when using chlorobutanol as the preservative in D5W. Span 20 was different from Tween 40 (p < 0.001) and Tween 80 (p < 0.001), while Tween 40 and Tween 80 were no different (p = 0.996). Therefore, rivastigmine is not emulsifiable by Span 20 whether the continuous phase is D5W or 0.9% NaCl. Microneedle dosage form is pain-free, minimal invasive and can be administered by health care professionals in clinics and hospital settings, trained home care personnel, or patients themselves at home. This is due to the short injection time to administer 0.5 to 2 mL without having to use a battery or electrical power (Figure 1a and 1b). Syringe filtration of 0.2 micron was applied to obtain the required sterilization of project formulations. Vacuum filtration may be tested as the first sterilization strategy in scale up due to the small volume per dose before studying another method. Future investigation can also focus on whether a preservative in the formulation of this single dose sealed product can be omitted. In the dermis dendritic cells function as immune system responses. Therefore, there will be great potential to deliver vaccines or large molecules using different subtypes of microneedles into the dermis, especially for pediatric, geriatric and other special needs patient populations.
References
- Alzheimer’s Association (2019) Alzheimer’s Disease Facts and Figures. Alzheimers Dement 17-57.
- https://www.accessdata.fda.gov/scripts/cder/ob/search_product.cfm (Accessed Nov 22, 2020).
- Exelon Scientific Discussion. Available at: https://www.ema.europa.eu/en/documents/scientific-discussion-variation/exelon-h-c-169-x-0038-epar-scientific-discussion-extension_en.pdf (accessed Nov 22, 2020).
- Birks JS, Chong LY and Grimley Evans J. (2015). Rivastigmine for Alzheimer’s disease. Cochrane Database Syst Rev. [crossref]
- Cummings J, Lefèvre G, Small G, Appel-Dingemanse S (2007) Pharmacokinetic rationale for the rivastigmine patch. Neurology [crossref]
- Sadowsky CH, Micca JL, Grossberg GT, Velting DM (2014) Rivastigmine from capsules to patch: therapeutic advances in the management of Alzheimer’s disease and Parkinson’s disease dementia. Prim Care Companion CNS Disord. [crossref]
- Donnelly RF, Singh TRR, Morrow DIJ, Woolfson MAD (2012) Microneedle-Mediated Transdermal and Intradermal Drug Delivery. Wiley.
- Dharadhar S, Majumdar A, Dhoble S and Patravale V (2019) Microneedles for transdermal drug delivery: a systematic review. Drug Development and Industrial Pharmacy 45: 188-201. [crossref]
- Larraneta E, Lutton RE, Woolfson DA and Donnelly RF (2016). Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Materials Science and Engineering R 104: 1-32.
- Corrie SR, Kendall MAF (2017) Transdermal Drug Delivery. In: Hillery A, editor. Drug Delivery: Fundamentals and Applications, CRC Press, pg: 225-226.
- 3: Physical and Physicochemical Principles of Drug Formulation, and Ch. 18 Emulsions. (2018). In: Fahr A, Voigt’s Pharamaceutical Technology . Wiley, pg: 41-42, 549-550.
- https://multimedia.3m.com/mws/media/1004089O/solid-microstructured-transdermal-system-smts-sell-sheet.pdf (Accessed Nov 22, 2020)
- S. Pharmacopeial Convention (2020) USP Monographs: Rivastigmine. In: USP43-NF38. Rockville MD: U.S. Pharmacopeia, pg: 3922.
- Scientific Discussion (2007). London: EMEA. https://www.ema.europa.eu/en/documents/scientific-discussion-variation/exelon-h-c-169-x-0038-epar-scientific-discussion-extension_en.pdf (Accessed Nov 22, 2020).