DOI: 10.31038/CST.2018331
Abstract
Introduction
Malignant pleural effusion is a sign of advanced disease with poor prognosis. The function of natural killer (NK) cells is to identify and destroy target tumor cells. This study aims to evaluate the role that cytotoxic NK subpopulations play when diagnosing malignant pleural effusion.
Methods
NK subpopulations were determined in pleural fluid and peripheral blood by flow cytometry in 71 patients who had suffered pleural effusion of unknown etiology. They were classified into three groups according to their final diagnosis: malignant, paramalignant and benign.
Results
The NK CD56 dim CD16- subpopulation in peripheral blood was the highest subpopulation in benign than in malignant or paramalignant cases (18.5% vs. 5.5% or 5.6%; p<0.001). Cytotoxic subpopulations NK CD56 dim CD16 + and NK CD16+ were higher in malignant and paramalignant than in benign cases (NK CD56 dim CD16+: 90.7% and 90% vs. 81.4%; p<0.001; NK CD16+: 95% and 95.6% vs. 86.5%; p<0.002). No differences were found in any cells studied in pleural fluid.
Conclusions
The data from this study suggested that determining the percentage of subpopulations NK CD56 dim CD16+ and NK CD16+, which perform an antibody-dependent cytotoxic function in peripheral blood, can be useful to diagnose malignant pleural effusion.
Keywords
diagnosis; flow cytometry; natural killer cells; natural killer subpopulations; pleural effusion; malignant
Introduction
Malignant pleural effusion (MPE) is a common clinical problem among patients with neoplastic disease. It is a sign of advanced disease associated with symptoms deteriorating and worse quality of life, with mean survival varying between 3 and 12 months [1]. Given its poor prognosis and clinical involvement, diagnoses must be made early. However, a malignancy diagnosis is not always possible with cytology, whose sensitivity range is 40–87% [2]. Hence the need to resort to complementary methods to identify tumor cells within pleural effusions (PE), [3] and to start early therapeutic interventions in an attempt to reduce these patients’ morbimortality.
MPE are characterized by a high percentage of mononuclear cells involved in immunological defense mechanisms, natural killer (NK) cells being one of the main components of the immunological system that participate in anti-tumoral defense mechanisms [4]. In theory, the presence of a high percentage of NK cells in pleural fluid could help establish its neoplastic nature. However, total NK (CD3- CD56+) quantification in pleural effusions has provided contradictory results in former studies [5–9].
Nowadays, there is very little information about the different NK cell subpopulations which can be found in MPE. However, it is known that the function of these subpopulations can be identified using the intensity of the expression of CD56 and CD16 surface antigens [10]. NK CD56 bright are considered regulator cells given their high capacity of producing pro-inflammatory and anti-inflammatory cytokines, [11] while NK CD56 dim [12] are cytotoxic given their high lytic activity. If the latter are also accompanied by a high CD16 expression, this makes them efficient mediators of antibody-dependent cell cytotoxicity [13]. CD57+ expression is a marker with a strong cytotoxic potential [14,15]. However, there is no data available on CD57+ expression in MPE. Therefore, the already available data seems to indicate that determining only total NK cells is not enough to identify MPEs differentiate them from benign ones. As NK subpopulations are characterized by performing more specific functions, the objective of this study was to study NK cell subpopulations, mainly those with a cytotoxic function, and their discriminative power in early differentiation of MPE, paramalignant pleural effusions (PPE) and benign pleural effusions (BPE).
Methods And Materials
Subjects
This two year (January 2013 to February 2015) prospective observational cohort study included 73 patients who had suffered pleural effusion of unknown etiology and were to undergo diagnostic thoracentesis. The final sample included 71 patients, two patients were excluded as no cellularity was obtained in pleural fluid. Patients were classified into three well differentiated groups according to their PE diagnosis: MPE, PPE and BPE (Figure 1).
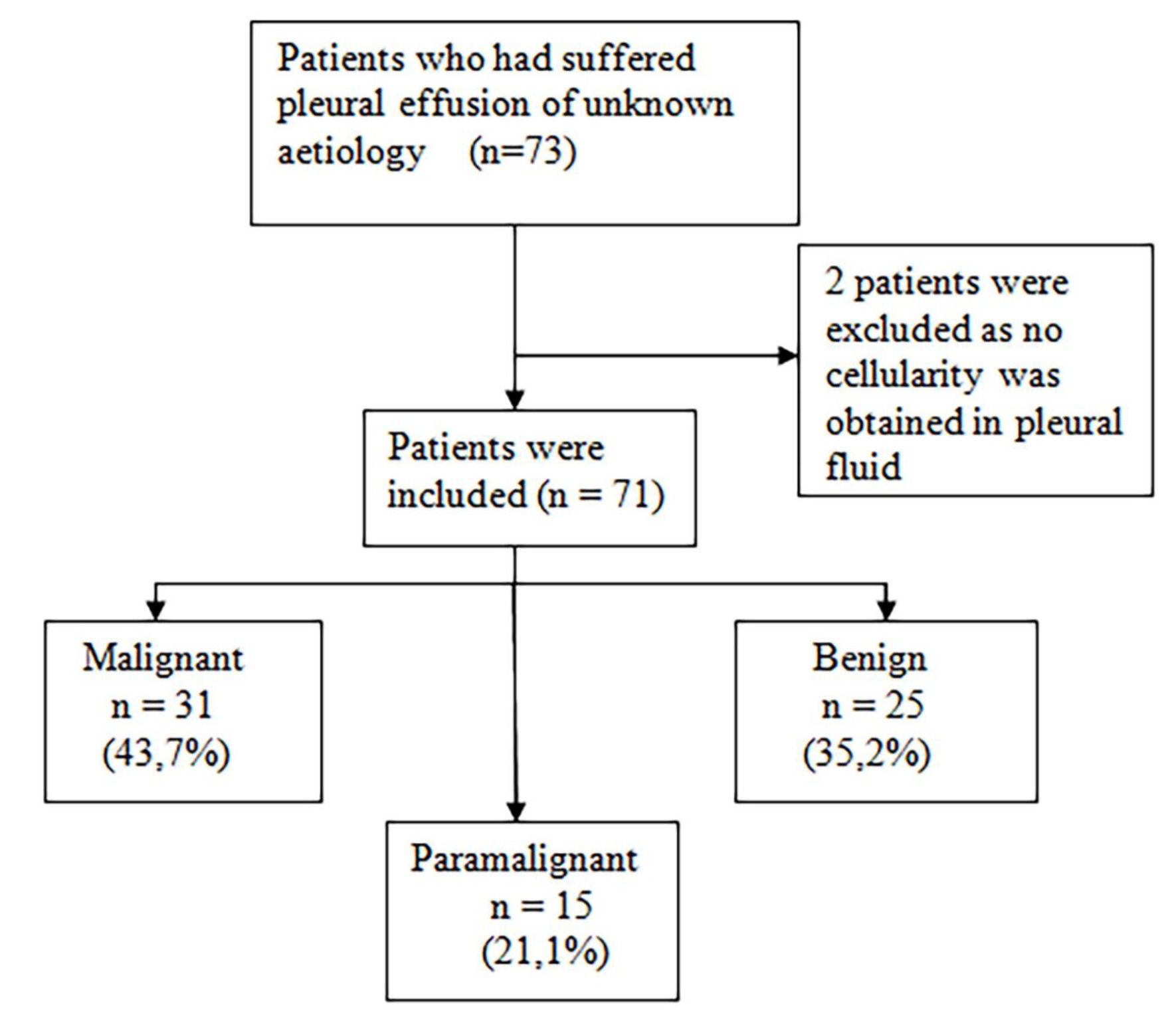
Figure 1. Flow of patients included in the study
Diagnosis of the type of PE was done according to the following criteria:
MPE was diagnosed if the presence of tumor cells in the pleural cavity was confirmed by a cytological study of pleural fluid, or in pleural tissue obtained by blind pleural biopsy, thoracoscopy or thoracotomy.
PPE (16) is due to a tumor process, but with no direct pleural infiltration by the tumor, and no tumor cells in the pleural fluid or tissue can be determined.
A PE is considered a BPE or as non-specific, when tumor etiology has been reasonably ruled out by imaging techniques, previous examinations, medical history and patient follow-up.
This study complies with the principles of the Declaration of Helsinki. Ethical approval of the study was given by Committee of Ethics and Clinical Trials (CEIC) of the Dr. Peset University Hospital in Valencia, with CEIC code: 10/12 on 29 February 2012. All the participating patients received written information about the nature and purposes of the study and gave their informed consent. A prospective follow-up of patients’ progress was done until they died or the study ended. All patients who were asked to be included in the study agreed to participate.
Measuring Natural Killer Cells
The lymphocyte populations in both the pleural fluid obtained from the first diagnostic thoracentesis performed on each patient and in the peripheral blood taken on the same day were analyzed.
After extraction, homogenization of the peripheral blood sample is immediately performed by the stirrer and mixer The Coulter Mixer (Coulter Electronics Limited, Northwell Drive, Luton, Bedfordshire, LU3 3RH, England®). Then, in a polypropylene tube, 100 μl of sample is introduced with 10 μl of each of the chosen monoclonal antibodies: CD45, CD19, CD3, CD56, CD16 and CD57. The mixture will be incubated for 15 minutes in the dark at room temperature. 0.5ml of the erythrolytic solution OptiLyse® are added, vortexed (Super-Mixer, Lab-Line Instruments Inc.®) and re-incubated in the dark at room temperature for another 15 minutes. After incubation, 2 ml of phosphate-buffered saline are added, centrifuged for 5 minutes at 300 x g (~1,600 r.p.m.) in a Microcen 21® and finally the supernatants are decanted and the cells re-suspended in 1 ml of phosphate-buffered saline and then introduced into a Navios® flow cytometer (Beckman-Coulter).
As in peripheral blood, the pleural fluid sample requires homogenizing the sample after extraction performed using the stirrer and mixer The Coulter Mixer (Coulter Electronics Limited, Northwell Drive, Luton, Bedfordshire, LU3 3RH, England®). However, the pleural fluid sample must be enriched prior to incubation. To do this, 2 ml of phosphate-buffered saline are added to 2 ml of pleural fluid, shaken and centrifuged at 300 x g (~1,600 r.p.m.) in a Microcen 21® for 5 minutes. The supernatants are then decanted and the cells are re-suspended in 0.5 ml of phosphate-buffered saline. After this process, the incubation with the monoclonal antibodies and procurement of the sample to be introduced in the flow cytometer can be performed following the same steps as in the peripheral blood.
A blind analysis of the diagnosis was run with the Kaluza 1.3 software (Beckman-Coulter). The sensitivity of the technique was 10-2 –10-3. After the expression of CD45, B (CD19+ CD3-) and T (CD3+ CD19-) lymphocytes as well as NK cells (CD3- CD56+) were studied first and compared to the 100% total lymphocytes. After and according to the intensity of the expression of antigens CD56 and CD16, the following subpopulations were differentiated: NK CD56 bright (++) CD16-, NK CD56 bright (++) CD16+, NK CD56 dim (+) CD16-, NK CD56 dim (+) CD16+ and NKCD16+ (CD56+/++ CD16+). NKCD57+ (CD56+/++ CD57+) were also determined and percentage quantification was done of all the NK subpopulations compared to the percentage of total NK cells.
Statistical analysis
All the results obtained were analyzed using the Kolmogorov-Smirnov test for a sample to determine if they followed a normal distribution pattern. Results were compared using the chi-square test for qualitative variables, the Student’s t-test for parametric quantitative variables and the Mann-Whitney U test for non-parametric quantitative variables. When comparing more than two groups, a one-way ANOVA (analysis of variance) was applied to the parametric variables and the Kruskal-Wallis test to the non-parametric variables.
The diagnostic efficacy of the analysis of the cells from pleural fluid and peripheral blood which presented differences considered significant enough to discriminate between MPE/PPE and BPE was determined by a receiver operating characteristic (ROC) curve analysis with the area under the ROC curve (AUC). A p-value <0.05 was considered significant and their 95% confidence intervals (95% CI) were calculated by standard techniques. The statistical package IBM SPSS Statistics for Windows (version 21.0. Armonk, New York: IBM Corp., USA) was employed.
Results
Demographics
This study took place at the University Dr. Peset Hospital in Valencia from 2013 to 2015 and analyzed 71 patients who had suffered PE of unknown etiology. The study population’s mean age was 69.1 years, and no differences were observed among groups. Male gender clearly predominated among the MPE and PPE cases (Table 1). According to the final PE diagnosis made, three groups were formed: MPE, PPE and BPE (Figure 1). All the MPE were exudates as well as 93.3% of PPE and 80% of the BPE (p=0.027) Adenocarcinoma was the most frequent histology found among the MPE (Table 1).
Table 1. Characteristics of the patients with malignant, paramalignant and benign pleural effusions.
|
|
Malignant (n=31) |
Paramalignant (n=15) |
Benign (n=25) |
p-valueb |
|
Age (years) 95% CI |
69.2±8.9 65.9–72.4 |
69.8 ±11.1 63.6–76 |
68.7 ±12.2 63.6–73.7 |
0.949
|
|
Gender |
|
|
|
0.133 |
|
Male |
19 (61.3%) |
12 (80%) |
12 (48%) |
|
|
Female |
12 (38.7%) |
3 (20%) |
13 (52%) |
|
|
Diagnosis |
||||
|
|
Adenocarcinoma 22 (71%) |
|
Non-specific 12 (48%) |
|
|
|
Lymphoma 4 (13%) |
|
CHF 4 (16%) |
|
|
|
Mesothelioma 2 (6.5%) |
|
Infectious 3 (12%) |
|
|
|
Epidermoid 1 (3.2%) |
|
TBC 2 (8%) |
|
|
|
Microcytic 1 (3.2%) |
|
Exp. to asbestos 2 (8%) |
|
|
|
Myxoid sarcoma 1 (3.2%) |
|
Cirrhosis 1 (4%) |
|
|
|
|
|
RA 1 (4%) |
|
Abbreviations: CI (confidence interval), CHF (congestive heart failure), TBC (tuberculosis), Exp. (exposure), RA (rheumatoid arthritis).
aData expressed in absolute values and percentages or mean±SD.
bChi-square test or ANOVA.
Lymphocyte populations in pleural fluid and peripheral blood
Lymphocyte populations were studied by determining B and T lymphocytes and NK cells in pleural fluid and peripheral blood. No differences between the expression of any cell line of the different groups was observed; that is, NK cells showed no higher expression in any pleural effusion type.
NK subpopulations in pleural fluid and peripheral blood
NK subpopulations were analyzed according to the intensity of the expression of surface antigens CD56 and CD16. No differences were found between the MPE, PPE and BPE groups in any cells studied in pleural fluid. Surprisingly, in peripheral blood, significant differences between the groups in the NK subpopulations were found. Subpopulation NK CD56 dim CD16- was higher in BPE cases than in the MPE or PPE ones (18.5% vs. 5.5% or 5.6%; p<0.001). Cytotoxic subpopulations NK CD56 dim CD16 + and NK CD16+ were higher in the MPE and PPE cases than in BPE ones (NK CD56 dim CD16 +: 90.7% and 90% vs. 81.4%; p<0.001 and NK CD16+: 95% and 95.6% vs. 86.5%; p<0.002) (Table 2).
Similarly, NK subpopulations analysis in peripheral blood showed that subpopulation NK CD56 dim CD16- was higher in BPE cases (18.5% vs. 5.5%; p<0.001), and subpopulations NK CD56 dim CD16 + and NK CD16+ appeared mostly in the combined MPE and PPE group, and in the isolated MPE cases (NK CD56 dim CD16 +: 90.7% vs. 81.4%; p<0.001 and NK CD16+: 95% vs. 86.5%: p<0.002) (Table 3).
Table 2. Natural killer subpopulations in peripheral blood.
|
|
Malignant (n=31) |
Paramalignant (n=15) |
Benign (n=25) |
p-valueb
|
|
NK (CD3-CD56+) |
11.6 (0.7–73.2) |
9.6 (1.7–16.2) |
7 (0.7–31.3) |
0.520 |
|
NK CD56 bright |
0.5 (0–12.7) |
1.4 (0–9.1) |
0.5 (0–31) |
0.479 |
|
CD56 bright CD16- |
0.2 (0–2.7) |
0.4 (0–2) |
0.3 (0–17.8) |
0.720 |
|
CD56 bright CD16+ |
0.1 (0–11.4) |
0.7 (0–8.1) |
0 (0–13.2) |
0.155 |
|
NK CD56 dim |
98.6 (81.5–100) |
96.9 (91.6–100) |
99.4(65.9–100) |
0.189 |
|
CD56 dim CD16- |
5.5 (0.3–92.1) |
5.6 (0.3–24.4) |
18.5(2.5–100) |
0.001*** |
|
CD56dim CD16+ |
90.7 (7.4–99) |
90(70.7–99) |
81.4 (0–95.4) |
0.001*** |
|
NK CD16+ |
95 (7.8–99.6) |
95.6 (76.1–99.5) |
86.5 (0–97.1) |
0.002** |
|
NK CD57+ |
48.6±20 |
49.2±17.4 |
54.4±14.5 |
0.454 |
Abbreviations: NK (natural killer).
aPercentage data expressed as mean±SD or median (minimum-maximum).
bANOVA or Kruskal-Wallis test.
*p<0.05; **p<0.01; ***p<0.001
Table 3. Cytotoxic natural killer subpopulations in peripheral blood.
|
|
Malignant (n=31) |
Benign (n=25) |
p-valueb
|
|
CD56dim CD16- |
5.5 (0.3–92.1) |
18.5 (2.5–100) |
0.001*** |
|
95%CI |
2.3–13 |
11.8–28.1 |
|
|
CD56dim CD16+ |
90.7 (7.4–99) |
81.4 (0–95.4) |
0.001*** |
|
95%CI |
87–97.7 |
71.9–88.2 |
|
|
NK CD16+ |
95 (7.8–99.6) |
86.5 (0–97.1) |
0.002** |
|
95%CI |
92.8–99.8 |
78.8–92.9 |
|
Abbreviations: NK (natural killer) CI (confidence interval).
aPercentage data expressed as median (minimum-maximum).
bStudent’s t-test or Mann-Whitney U test.
*p<0.05; **p<0.01; ***p<0.001
Diagnostic efficacy of cytotoxic NK subpopulations
These results reveal that, despite there being no differences in the NK subpopulations in pleural fluid to differentiate malignant cases from benign ones, differences appeared in the following NK subpopulations in peripheral blood: NK CD56 dim CD16-, NK CD56 dim CD16 + and NK CD16+. In order to determine the diagnostic efficacy of the analysis of these subpopulations in blood, a ROC curve analysis with AUC was performed. The isolated determination of the percentage in peripheral blood of subpopulation NK CD56 dim CD16- had an AUC of 0.777 to discriminate a BPE from a MPE (95%CI: 0.653–0.901; p<0.001). If the cut-off point was 9.82%, sensitivity would be 76% and specificity would be 71%. In order to differentiate a BPE from a MPE/PPE, the AUC was 0.784 (95%CI: 0.671–0.897; p<0.001), with a sensitivity of 76% and a specificity of 72% with the same cut-off point (Figure 2).
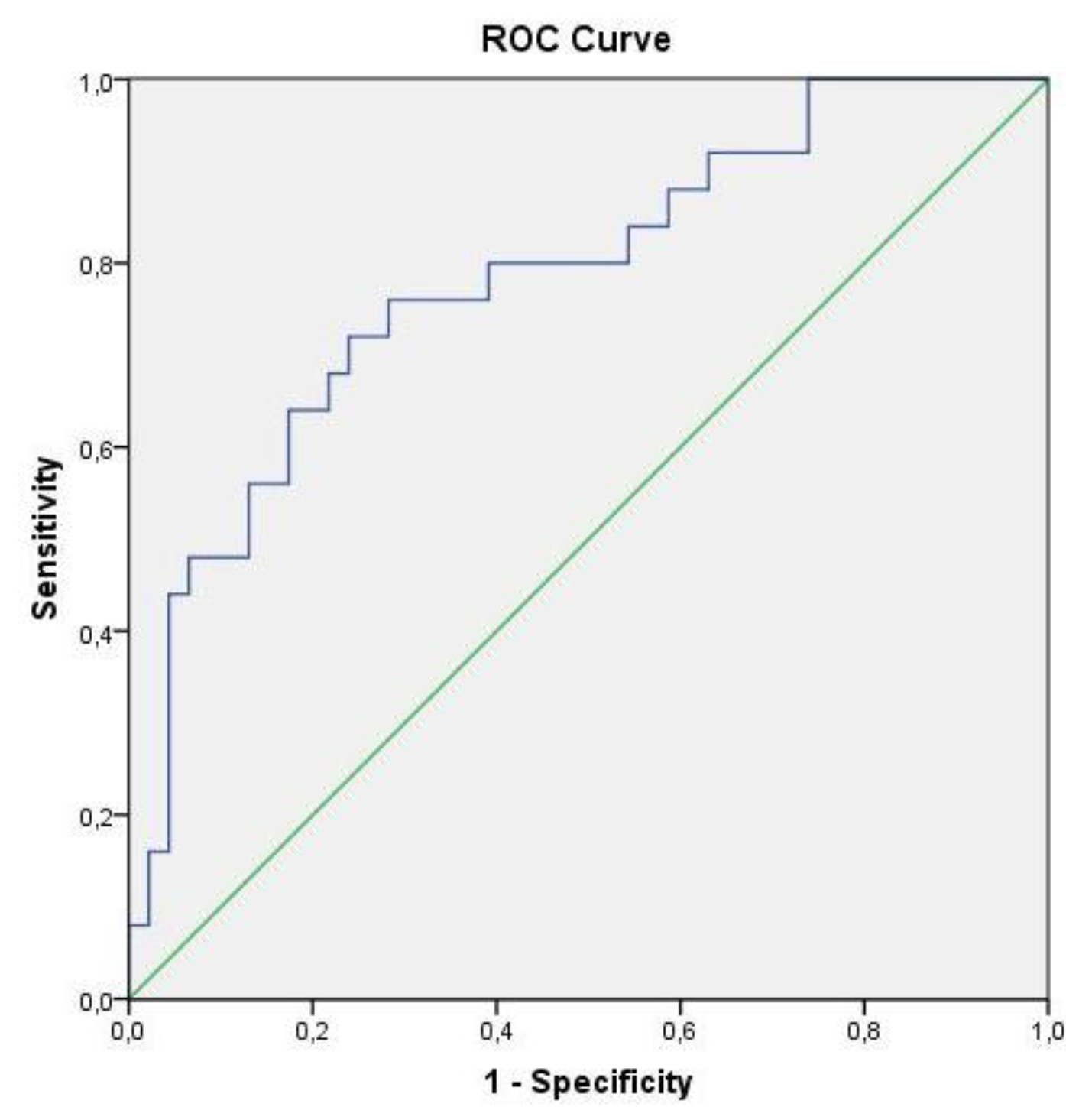
Figure 2. ROC curve of subpopulation NK CD56 dim CD16- to differentiate benign pleural effusions from malignant and paramalignant ones
Subpopulations NK CD56 dim CD16 + and NK CD16+, which have an antibody-dependent cytotoxic function, allow for discrimination of a patient with MPE from one with a BPE with an AUC of 0.761 (95%CI: 0.637–0.885; p=0.001) and 0.747 (95%CI: 0.619–0.876; p=0.002), respectively. In order to differentiate a MPE and PPE from a BPE, the AUC was 0.774 (95%CI: 0.663–0.885; p<0.001) and 0.753 (95%CI: 0.640–0.867; p<0.001), respectively (Figure 3).
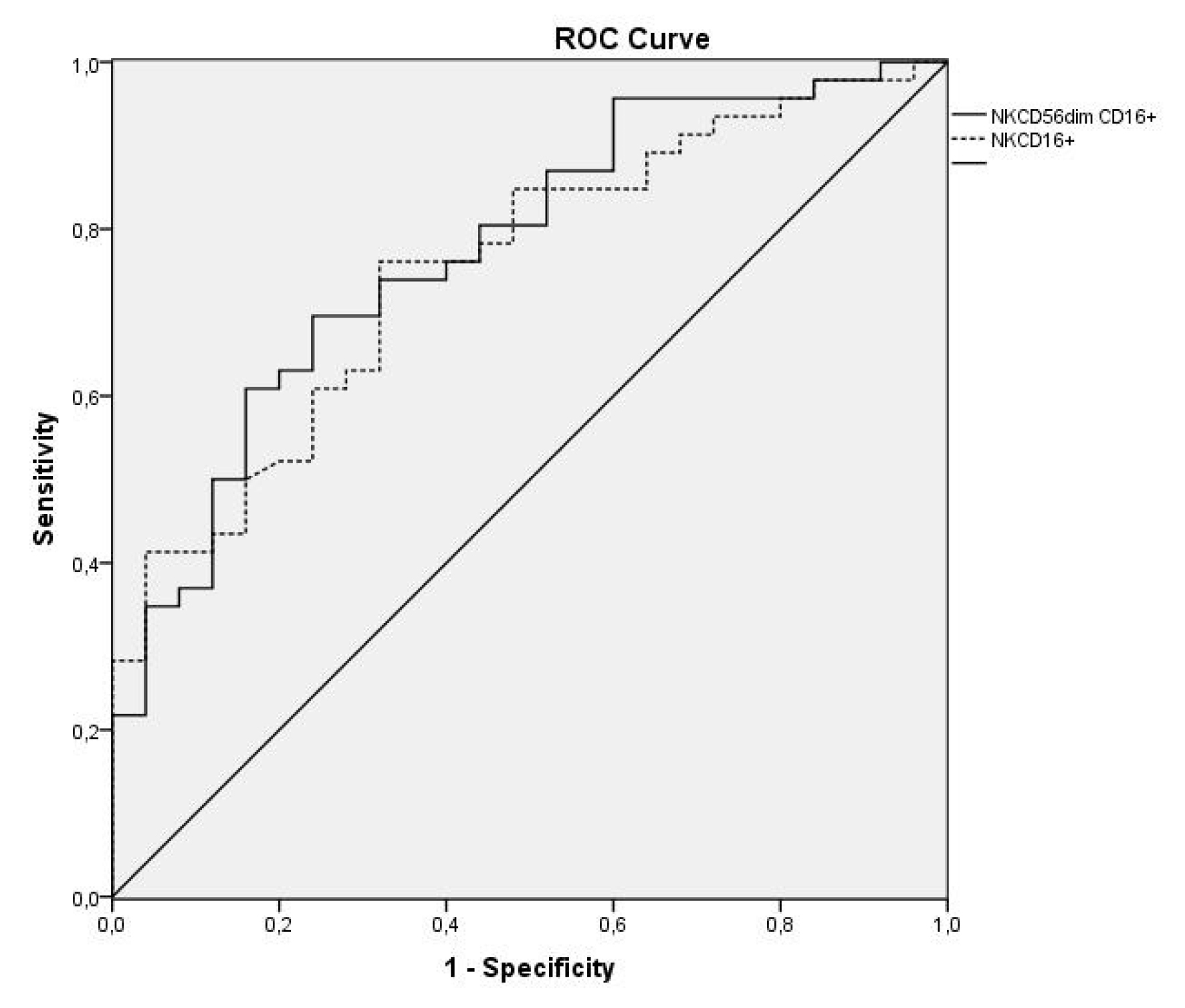
Figure 3. ROC curves of subpopulations NK CD56 dim CD16 + and NK CD16+ to differentiate malignant and paramalignant pleural effusions from those of a benign type
Discussion
MPE is a sign of advanced neoplastic disease which implies the pleural space has been affected by this malignant process. Given these patients’ poor prognosis, its diagnosis is therefore essential, and it would be very useful to identify markers that increase the possibility of diagnosing this malignity. Here, NK cells can play a key role in the defense against neoplastic invasion of the pleural cavity. In theory, detecting a high percentage of NK cells in MPE could help establish their tumoral nature. Despite some authors having observed a higher NK cell percentage in MPE, [5–7] others have reported a lower percentage, [8] and some groups, including our own, have not even found any differences [9]. It would appear that published data may indicate that determining only total NK cells is not sufficient to distinguish MPE from BPE. Therefore, we have centered our research on the NK subpopulations characterized by playing a cytotoxic function as presence of neoplastic cells in pleural fluid or tissue should reflect increased cytolytic activity in MPE compared to those of other etiologies. Apart from the subpopulations that explain the intensity of the expression of CD56 and CD16, potentially cytotoxic subpopulation NK CD57+ was also evaluated in differentiating a MPE from a BPE. Our data demonstrated that although no differences between groups or between malignant and benign cases were found in any of the studied cells in pleural fluid, differences appeared in peripheral blood: subpopulation CD56 dim CD16- was higher in BPE cases, and subpopulations CD56 dim CD16 + and NK CD16+ were higher in MPE and PPE ones. This indicated that a high CD16 expression made them efficient mediators of antibody-dependent cell cytotoxicity [13] with expressions in blood, but not in pleural fluid. These findings led us to wonder if there was a more relevant systemic response than the local one in patients with MPE. No published works have analyzed the diagnostic value of NK subpopulations in peripheral blood to distinguish between MPE and BPE. Moreover, information about pleural fluid is scarce. Scherpereel et al.[17] found increased CD16+ in pleural fluid in all PE except for BPE. Cornfield et al.[4] analyzed 30 malignant (pleural, pericardial and ascitic) effusions and 30 benign ones, and found no differences in the percentage of any subpopulation they studied. These authors only reported an increase in the absolute value of NK CD16+ in malignant effusions. Pace et al.[18] encountered that NKCD16+ percentages in patients with MPE and BPE were similar. The comparison made of the findings from this work with the few existing studies is complicated due to the different methodologies employed. Our data coincide with those reported by Cornfield et al. [4] The work by Pace et al. [18] only included 19 patients with MPE, while the BPE group differed (due to heart failure) to that herein studied as they were effusions of unknown etiology suspected of malignity, which pose a problem in diagnosing MPE. Moreover, although NKCD57+ displayed high cytolytic activity, [14,15], no published studies have been conducted on this marker in MPE, and this is the first work to analyses it.
In order to determine the diagnostic efficiency of the analyses of these subpopulations in blood, a ROC curve analysis was carried out. The subpopulation with the largest AUC to differentiate BPE from malignant ones was NK CD56 dim CD16- (0.777), which increased to 0.784 when the discrimination was between BPE and both MPE and PPE. When distinguishing between malignant and benign cases, subpopulations NK CD56 dim CD16+ and NK CD16+ had an AUC of 0.761 and 0.757, respectively. When MPE and PPE were distinguished from BPE the AUC increased to 0.774 and 0.753. This result has never been previously reported.
The main limitation of this study was that 21.1% of the included effusions were of the PPE type. Other studies[4,18] did not include this type. However, as the PPE type has its typical characteristics and is associated with poor prognosis, we decided to include it to well reflect the usual clinical reality.
By way of conclusion, determining the percentage of NK cells in pleural fluid of PE of unknown etiology does not allow malignant cases to be differentiated from benign ones. However, determining the percentage of subpopulations NK CD56 dim CD16+ and NK CD16+ that perform an antibody-dependent cytotoxic function in peripheral blood was identified as a diagnostic test whose capacity helps to early discriminate a patient with a MPE.
Author Contributions: All authors have been involved in the conception and design, or analysis and interpretation of data, as well as in drafting the article or revising it critically for important intellectual content. Maria Morales-Suarez-Varela has been designated as guarantor for the article.
Acknowledgements: The authors would like to thank the Pulmonology Foundation of the Valencian Community for the grant this work was awarded with, and with which the monoclonal antibodies employed were obtained. They would also like to thank everyone who has collaborated either directly or indirectly in this research.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
Competing Interest: The authors declare that they have no competing interest.
Funding Information: Dr. Herrera Lara has received research scholarship support from the Pulmonology Foundation of the Valencian Community.
References
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ (2010) BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline Thorax 65 Suppl 2: ii32–40.
- Villena Garrido V, Ferrer Sancho J, Hernández Blasco L, de Pablo Gafas A, Pérez Rodríguez E, Rodríguez Panadero F, et al. (2006) Diagnóstico y tratamiento del derrame pleural. Arch Bronconeumol 42: 349–372.
- Stonesifer KJ, Xiang JH, Wilkinson EJ, Benson NA, Braylan RC (1987) Flow cytometric analysis and cytopathology of body cavity fluids. Acta Cytol 31: 125–130.
- Cornfield DB, Gheith SM (2009) Flow cytometric quantitation of natural killer cells and T lymphocytes expressing T-cell receptors alpha/beta and gamma/delta is not helpful in distinguishing benign from malignant body cavity effusions. Cytometry B Clin Cytom 76: 213–217.
- Green LK, Griffin J (1996) Increased natural killer cells in fluids. A new, sensitive means of detecting carcinoma. Acta Cytol 40: 1240–1245. [crossref]
- Yu GH, Hida CA, Salhany KE, Baloch Z, Gupta PK (1999) Immunohistochemical detection of cytotoxic lymphocytes in malignant serous effusions. Diagn Cytopathol 21: 18–21.
- Laurini JA, Garcia A, Elsner B, Bellotti M, Rescia C (2000) Relation between natural killer cells and neoplastic cells in serous fluids. Diagn Cytopathol 22: 347–350.
- Sikora J, Dworacki G, Trybus M, Batura-Gabryel H, Zeromski J (1998) Correlation between DNA content, expression of Ki-67 antigen of tumor cells and immunophenotype of lymphocytes from malignant pleural effusions. Tumor Biol 19: 196–204.
- Jezewska E, Sikora J, Slowik-Gabryelska A, Zeromski J (1993) Evaluation of immunophenotype of lymphoid cells isolated from malignant pleural effusions. Arch Immunol Ther Exp (Warsz) 41: 51–56.
- Caligiuri MA (2008) Human natural killer cells. Blood 112: 461–469. [crossref]
- Poli A, Michel T, Thérésine M, Andres E, Hentges F, Zimmer J (2009) CD56bright natural killer (NK) cells: an important NK cell subset. Immunology 126: 458–465.
- Sedlmayr P, Schallhammer L, Hammer A, Wilders-Truschnig M, Wintersteiger R, Dohr G (1996) Differential phenotypic properties of human peripheral blood CD56dim and CD56bright natural killer cell subpopulations. Int Arch Allergy Immunol 110: 308–313.
- Montserrat Sanz J, García Torrijos C, Díaz Martín D, Prieto Martín A (2013) Linfocitos natural killer. Medicine(Spain) 11: 1728–1736.
- Lopez-Verges S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, et al (2010) CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood 116: 3865–3874.
- Nielsen CM, White MJ, Goodier MR, Riley EM (2013) Functional Significance of CD57 Expression on Human NK Cells and Relevance to Disease. Front Immunol 4: 422.
- American Thoracic Society (2000) Management of malignant pleural effusions. Am J Respir Crit Care Med 1987–2001.
- Scherpereel A, Grigoriu BD, Noppen M, Gey T, Chahine B, Baldacci S, et al. (2013) Defect in recruiting effector memory CD8 T-cells in malignant pleural effusions compared to normal pleural fluid. BMC Cancer 13: 324.
- Pace E, Di Sano C, Ferraro M, Tipa A, Olivieri D, Spatafora M, et al. (2011) Altered CD94/NKG2A and perforin expression reduce the cytotoxic activity in malignant pleural effusions. Eur J Cancer 47: 296–304.