DOI: 10.31038/AFS.2022433
Abstract
Wheat is important crop in globally both in production and productivity. It is sourced as food for global population. Due to un-matched wheat production and population growth it is not able to meet the feed. The low production and productivity it is due to fungal disease specially rust (stem rust) disease. The objective of the experiment was to identify and detect Pgt across surveyed areas. The experiment was conducted in 2019/20 main rainy season East Shewa zone, North Shewa zone and Hadiya Zones which has high wheat production potential with suitability environment for the disease development. A total of fifty five infected wheat stem rust samples were collected from the three regions. Only 35 isolates have given produced infectious pustules. Races: TKTTF, TTKTF, TTTTF, TKPTF, TKKTF and TTKTT have been identified. TTTTF, TKTTF and TKKTF are found at all assessed regions of Ethiopia. The collected samples were analyzed at Ambo Agricultural Research Center (AmARC) laboratory for race identification.
Keywords
Distribution, Cultivars, Diversity, Incidence, Races, Rust and severity
Introduction
Wheat is a cereal grains produced and consumed globally [1]. It is one of strategic crop for food security and a source of livelihood in emerging countries [2]. Wheat also remains a major source of dietary calories and proteins [2]. As a result, yield and its cultivation area of wheat have been increased to contribute to total production increase [3]. Ethiopia is experienced and largest wheat producer in sub-Saharan Africa with about 0.75 million hectare. Ethiopia can produce both bread and durum wheat cultivated in the highlands of the country largely in the areas like North West, East and Central parts [4]. However, the relations of yield and production in Ethiopia were not well understood in quantitative terms. At present, wheat is produced both in rain fed and irrigated conditions with an average yield of 2.764 t/ha in 2019 [5], but the yield is still lower the global wheat production about 3.56 t/ha. Wheat stem rust is the major constraints for wheat production in the world and in the Eastern part of Africa particularly Ethiopia and Kenya. Yield loss of stem rust reaches 100% in conducive environment and susceptible varieties during year of epidemics. Countries such as Kenya and Ethiopia experience recurrent epidemics of stem rust due to evolution of new stem rust races [6]. The alternate hosts of P. graminis include Berberis spp., Mahonia spp., P. recondita and Clematis [7]. The sexual cycle produces a great genetic diversity with a large number of virulence genes [8]. Evidence points to the recombination of wheat stem rust and the scabrum rust (P. graminis f. sp. secalis) [9, 10]. Puccinia graminis tritici, evolves and mutates, the popularly grown wheat varieties remain at constant stake of losing their resistance to break the strongest of resistant genes [11]. The first virulent stem rust race which was designated as Ug99 in Uganda in 1999 has threatened wheat production globally [12]. The emergence of new virulent races in East Africa are continue to pose a threat to global wheat production and food security [13]. Most highlands of Ethiopia are considered as a hot spot for the development of stem rust complex [14]. Stem rust race are dominant and widely distributed in different regions with high frequency [15]. Several historical events were happened in parts of Ethiopia in recent stem rust caused great losses: stem rust epidemics in 1975 on variety Laketch; in 1992/93, on variety Enkoy; in 1994, on variety Kubsa; and, in 2013, on variety Digelu. The epidemics are due to the appearance of new races as a result of mutation and sexual recombination. To minimize the threat of epidemics, it is important to characterize the race composition of the pathogens and the appearance of new races in the Ethiopia. So it is aimed to identify the Puccinia graminis f.sp. tritici physiological races distribution across different areas and identify seedling resistance test.
Materials and Methods
The field assessment was carried out during 2019/20 main cropping season in three major wheat growing areas such as East Shewa zone, North Shewa zone and Hadiya Zones which are selected based on wheat production potential and highly suitable environment for the disease development. During assessment farmer’s field, Farmer’s Training Center (FTC) and agricultural research wheat stations with different crop growth stages based on Zadoks growth stage (0-9) key (Table 1).
Table 1: Agro-ecological descriptions of areas
|
Zone |
Districts | Coordinates | Altitude (m.a.s.l) | Temperature (°C) | RF (mm) | ||
| N | E | Min. |
Max. |
||||
| East shewa zone | Ada’a |
08°44′′ |
38°58′′ | 1950 | 8°C | 28°C |
851 |
| Gimbichu |
08°58′′ |
39°06′′ | 2450 | 9°C | 29°C |
1200 |
|
| Lume |
08°12′′ |
39°17′′ | 1900 | 9.2°C | 29.3°C |
951 |
|
| Hadya zone | Lemo |
07°30′′ |
37055′′ | 2001 | 13°C | 26°C |
1150 |
| Misha |
07°56’′ |
38°52′′ | 2143 | 10.5°C | 22.5°C |
869 |
|
| Duna |
07°20′′ |
37°39′′ | 2453 | 12°C | 24°C |
932 |
|
| North shewa zone | Moret ena Jiru |
09°36′′ |
39°38′′ | 2828 | 6.1°C | 24°C |
890 |
| Basona werana |
10°41′′ |
39°47′′ | 2828 | 13.5°C | 21.5°C |
1000 |
|
| Minjar |
08°45′′ |
39°15′′ | 2120 | 13°C | 29°C |
854 |
|
Stem rust severity and incidence was made at five points along the two diagonals (in an “W” pattern) of the field using 1m x 1m (1m2) quadrant and used to calculate average values. The stem rust incidence was calculated using the number of infected plants and expressed as a percentage of the total number of plants assessed.
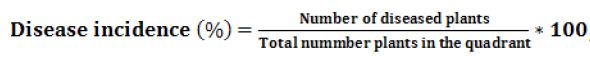
The disease severity was measured as a percentage of stem area infected by rust disease according to Modified Cobb’s scale (Figure 1). The severity of the disease was examined on randomly selected five plants in quadrant.
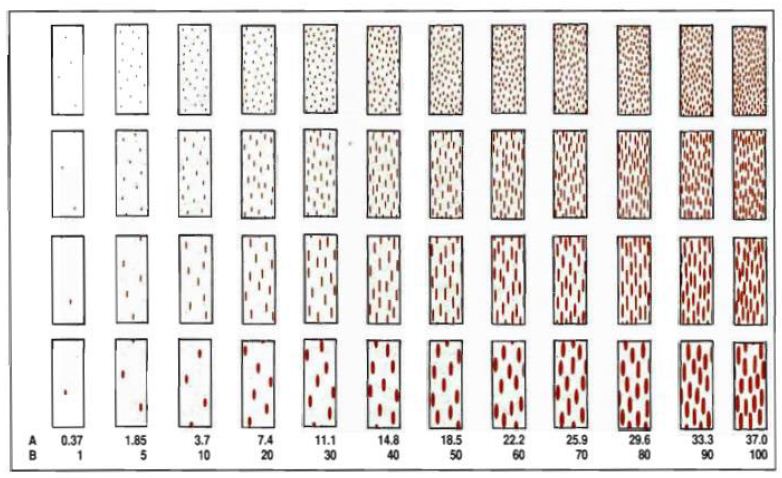
Figure 1: Rust severity estimation on leaves and stem of wheat. A. Percentage occupied by uredinia. B. Rust severities by Peterson et al. (1948). Source: Roelfs et al. (1992)
In addition to the disease parameters, agronomic and geographical data were recorded. Data on geographical information including latitude, longitude and elevation of each field were recorded using Garmin 600 model GPS.
Sample Collection, Isolation and Multiplication of Single-pustules Pathogen
Infected wheat stem sheath and leaf were cut into pieces of 5-10 cm in length using scissors then placed in paper bags. The collected samples were labeled with the zone, district, variety and date of collection then transported to Ambo Agricultural Research Center (AmARC) laboratory for race identification. Sterilized soil composed of three growing Medias; sand, soil and farmyard manure mixed at the ratio of 2:1:1 by volume were used. “McNair” seeds were raised in 5cm diameter pots by spreading the seeds on filter paper in Petri dishes, moisten with water to allow the radicles sprout and sprouted seeds were planted in to growing pots.
Spores collected from rust infected sample after suspension then inoculated onto a week old McNair seedlings [16]. The inoculated seedlings were placed on a table for 30 minutes until the Soltrol evaporate then the seedling is moistened with fine droplets of distilled water and placed in the incubation chamber in a dark at 18-22°C followed by exposure to light for 3-4 hours to facilitate infection. The humidifier switched on for about 1:30 hours, so the seedlings have enough moisture for the whole dark period to condition facilitate the initial infection. Then after, the seedlings were transferred to glass compartments in the greenhouse a temperature of 18-25°C and relative humidity of 60-70%.
Inoculation of Wheat Stem Rust Differential Host Lines
Five seeds of the twenty wheat stem rust differentials with known resistance genes and susceptible variety McNair were grown in 5 cm diameter pots. The required amount 4 mg of spore was prepared in 1ml lightweight mineral oil suspension and inoculated onto a week old seedlings of the differentials. Inoculated plants were moistened with fine droplets of distilled water and placed in an incubation chamber 18-22°C and 98-100% RH. Inoculated seedlings were placed in separate glass compartments greenhouse temperature adjusted with 18°C and 25°C. Natural daylight was supplemented with an additional 4 hrs/day that emitted by cool white fluorescent tubes arranged directly above plants (Figure 2) [7].

Figure 2: Picture captured during the sample collection on severely infected wheat varieties
Determination of Stem Rust Races
Seedling infection types (ITs) were scored 14 days after inoculation using 0 to 4 scoring scale described by [17]. The IT readings of 3 (medium-size uredia with/without chlorosis) and 4 (large uredia without chlorosis or necrosis) were regarded as susceptible. Other readings, i.e. 0 (immune or fleck), 1 (small uredia with necrosis) and 2 (small to medium uredia with chlorosis or necrosis) were resistant (Figure 3).
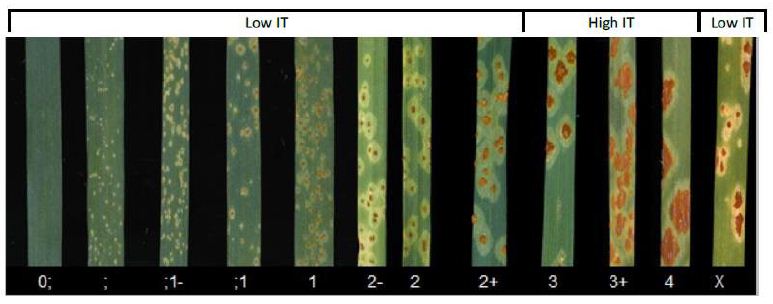
Figure 3: Infection types of Pgt and host response. Source: Stackman et al. (1962)
Race identification and designation was done using the North American’s nomenclature system for Pgt and grouped the differential lines into five subsets (Table 2). Each isolate was assigned five letter of designation code of [7]. Based on low IT′s isolate that produces on 20 differential lines; the race was designated with a five letter race code BBBBB. Conversely, an isolate that produces a high IT on the 20 differential lines races coded as TTTTT. If an isolate produces a low IT on Sr31 and Sr24, but high infection type on the remaining 18 differential lines, the race was designated as TTTTF.
Table 2: North American’s nomenclature system for Pgt differential wheat lines
|
Wheat Pgt gene differential sets and infection phenotype coding |
||||
|
Set |
Differential lines identified by Pgt resistance gene |
|||
| Set 1 |
5 |
21 | 9e |
7b |
| Set 2 |
11 |
6 | 8a |
9g |
| Set 3 |
36 |
9b | 30 |
17 |
| Set 4 |
9a |
9d | 10 |
Tmp |
| Set 5 |
24 |
31 | 38 |
McN |
| gt-code | Infection phenotype: High: virulent (susceptible); low=avirulent (resistant). | |||
| B |
Low |
Low | Low |
Low |
| C |
Low |
Low | Low |
High |
| D |
Low |
Low | High |
Low |
| F |
Low |
Low | High |
High |
| G |
Low |
High | Low |
Low |
| H |
Low |
High | Low |
High |
| J |
Low |
High | High |
Low |
| K |
Low |
High | High |
High |
| L |
High |
Low | Low |
Low |
| M |
High |
Low | Low |
High |
| N |
High |
Low | High |
Low |
| P |
High |
Low | High |
High |
| Q |
High |
High | Low |
Low |
| R |
High |
High | Low |
High |
| S |
High |
High | High |
Low |
| T |
High |
High | High |
High |
Source: [7]
Disease Data Collection
Seedling infection types (ITs) data obtained from the race analysis study was used for the identification of races using the North American′s nomenclature system for distribution of Pgt races percentage across the study area (zones), altitude ranges and cultivated varieties was analyzed using descriptive statistics by using Microsoft excel. Infection type data obtained from the seedling resistance analysis of the greenhouse experiment was used to group cultivars under different resistance categories according to [17]. Percentage of durum wheat cultivars having resistant vs. susceptible reaction to selected stem rust races was computed using descriptive statistics in by using Microsoft excel.
Result and Discussion
A total of fifty five infected wheat stem rust samples were collected from the three regions. Of those samples collected, 35 isolates have given produced infectious pustules, but 20 samples didn’t yield viable isolates. Of these isolates, 6 races namely TKTTF, TTKTF, TTTTF, TKPTF, TKKTF and TTKTT were identified (Figure 3). TTTTF, TKTTF and TKKTF are found at all selected regions of Ethiopia while; race TTKTF is only found at North shewa and Hadiya zone of wheat potential production area (Figure 4).
Figure 4: Distribution of Puccinia graminis f.sp. tritici races in assessed areas. Prevalence of puccinia graminis f.sp. tritici races
The diversified distributions of races were prevalent at northern shewa. Out of the samples 35 viable stem rust samples collected; TKKTF race is identified from 17 isolates at about 48.57%; while TTTTF is isolated 8 times. This implies that that TKKTF and TTTTF are the dominantly diversified races in the areas. The race variation at different location wheat varieties grown and environmental conditions [16, 18, 19]. TKKTF was identified form Hidase variety which is wiped out by this race in 2017/18. From the study indicated that race TTTTF was the second most diversified races in the study locations. The second most everyday identified races were TTTTF and TKTTF at a rate of 14.81% each. Race TKKTF was found at all surveyed locations except Ada’a district (Table 3).
Table 3: Amount of races isolated from assessed fields related to cultivated varieties
|
Race |
Field inspected | Percentage of races |
Variety |
| TKKTF |
17 |
30.91% |
Mangudo, Kubsa, Kakaba, Land races and Hidase |
| TTTTF |
8 |
14.55% |
Hidase, Kubsa, Mangudo and Tesfaye |
| TKTTF |
4 |
7.27% |
Mangudo and kubsa |
| TTKTF |
3 |
5.46% |
Kakaba and Kubsa |
| TTKTT |
2 |
3.64% |
Kubsa and Mangudo |
| TKPTF |
1 |
1.82% |
Uknown |
Altitudinal Variation Influence on Pgt Races Distribution
Altitudinal ranges have influence on distribution of stem rust races diversity. The result revealed that there are variations in stem rust incidence, severity levels and races as well. Much more in number about 22 races with the percentage of 62.86% were identified from high altitudes ranging from 2300-2863 m.a.s.l, conversely; lower number of races was identified at low altitudes ranging from 1500-2300 m.a.s.l. Wide ranges of incidence 0-100% was recorded at higher altitude between 2300-2863 m.a.s.l while the narrow incidence 20-100% was recorded at low altitude between 1500-2300 m.a.s.l (Table 4).
Table 4: Altitudinal variation influence on Pgt races distribution in 2018/2019
|
Altitude (m.a.s.l) |
No. of identified races | Percentage % | Incidence range | Severity range |
Host response |
| 1500-2300 |
13 |
37.14% | 20-100 | 5-70 |
MS-S |
| 2300-2863 |
22 |
62.86% | 0-100 | 0-70 |
MS-S |
| Mean |
17.5 |
50 | 0-100 | 0-70 |
MS-S |
Effect of Growth Stage on Pgt Races Distribution
Wheat stem rust distribution is affected by wheat growth and maturity stage depending on food accumulated and prepared in the host plant. Races about 21 in number were identified were detected at dough full growth stage in enough food accumulated (Table 5). Wider ranges of disease incidence 0-100% were recorded at dough stage, while narrow range disease incidence 15-80% was at milk stage. Higher rust severities were recorded at maturity stage 15-70%, but lower severity 5-40% was at milk stage. This implies that wheat growth and maturity stage have influences on rust epidemics.
Table 5: Effect of growth stage on Pgt races distribution in 2018/2019
|
Maturity stage |
No. of identified races | Percentage % | Incidence range | Severity range |
Host response |
| Milk |
9 |
25.71 | 15-80 | 5-40 |
MS-S |
| Dough |
21 |
60.00 | 0-100 | 0-70 |
MS-S |
| Maturity stage |
5 |
14.29 | 100 | 15-70 |
MS-S |
| Mean |
11.67 |
33.33 | 0-100 | 0-70 |
MS-S |
As shown in the graph below more races were detected on kubsa with high percentage record. Races about 14 times were identified sample collected kubsa bread wheat variety. Hidase is one of the popular varieties which is one of the severely infected currently about four races with a percentage of 11.43% (Figure 5) have been recorded on samples collected. One of detected variety is kakaba about 7 races has been identified with percentage of 20%. Among this lesser race about 1 and 2.86% was identified on Tesfaye variety. Mangudo is found to be the second variety infected by nine races with percentage 25.71% among others (Figure 5).
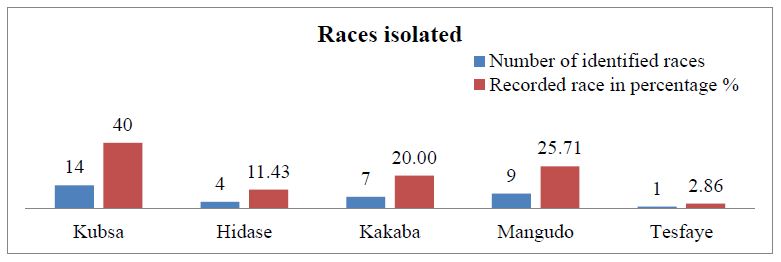
Figure 5: Number of races identified and recorded percentage
Summary and Conclusion
Thirty five stem rust isolates were analyzed on twenty stem rust differentials and six stem rust races namely: TKTTF, TTKTF, TTTTF, TKPTF, TKKTF and TTKTT are identified. Race TKPTF was prevailed only at East Shewa zone Ada’a districts, while races TKKTF and TTTTF were dominant isolate from all assessed zones of regions and collected samples which receive the first and second ranks of samples. This implies that TKKTF and TTTTF are the dominantly diversified races at surveyed fields at a rate of 48.15 and 14.55% respectively. Race TKKTF was found at all surveyed locations except Ada’a district. Much more in number about 22 races with the percentage of 62.86% were identified from high altitudes ranging from 2300-2863 m.a.s.l, conversely; lower number of races was identified at low altitudes ranging from 1500-2300 m.a.s.l. Wide ranges of incidence 0-100% was recorded at higher altitude between 2300-2863 m.a.s.l while the narrow incidence 20-100% was recorded at low altitude between 1500-2300 m.a.s.l. In addition to this agro-ecological variation and types of wheat varieties cultivated has greater influence in the presence of races. Survey and surveillance is required to clearly indicate the available Pgt race available in Ethiopia. To develop management methods for each races the breeder is intended to upgrade research on resistance protocols.
Acknowledgment
I would like to thank Ethiopian Institute of Agricultural Research and Debre Zeit Agricultural Research Center for the release of Budget.
Conflict of interest
The author has no declared any conflict of interest
References
- Igrejas G, Branlard G (2020) The importance of wheat. In Wheat quality for improving processing and human health.(1-7). Springer, Cham.
- Shiferaw B, Smale M, Braun HJ, Duveiller E, Reynolds M, et al. (2013) Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Security5: 291-317.
- Tesfaye B, Mesfin K, Tolera A, Gebresilasie H, Gebreyes G, et al. (2019) Some maize agronomic practices in Ethiopia: A review of research experiences and lessons from agronomic panel survey in Oromia and Amhara regions. African journal of agricultural research 14: 1749-1763.
- Addis Ababa Chamber of Commerce and Sectoral Associations (AACCSA) (2017) Value chain study on wheat industry in Ethiopia by afro-universal consultant and general trading P.L.C. final report Addis Ababa, January, 2017: 12-59.
- CSA (2019) Report on area and production of major crops (For private peasant holding, Meher Season Volume 1). Addis Ababa, Ethiopia: Central Statistical Agency.
- Eshete BB (2018) Status and challenges of wheat stem rust (Puccinia graminis F. sp. Tritici) and threats of new races in Ethiopia. Int J For Hortic 4: 22-31.
- Jin Y, Szabo LJ, Pretorius ZA, Singh RP, Ward R, et al. (2008) Detection of virulence to resistance gene Sr24 within race TTKS of Puccinia graminis f. sp. tritici. Plant Disease 92: 923-926. [crossref]
- Roelfs AP (1992) Rust diseases of wheat: concepts and methods of disease management. Cimmyt.
- Burdon JJ, Marshall DR, Luig NH (1981) Isozyme analysis indicates that a virulent cereal rust pathogen is a somatic hybrid. Nat 293: 565-566.
- Luig NH, Watson I (1972) The role of wild and cultivated grasses in the hybridization of formae speciales of Puccinia graminis. Aust J Biol Sci 25: 335-342.
- Ambika R, Meenakshi D (2018) Wheat Stem Rust Race Ug99: A Shifting Enemy. Int J Curr Microbiol App Sci 7: 1262-1266.
- Pretorius ZA, Singh RP, Wagoire WW, Payne TS (2000) Detection of virulence to wheat stem rust resistance gene Sr31 in Puccinia graminis f. sp. tritici in Uganda. Plant Dis 84: 203. [crossref]
- Olivera P, Newcomb M, Szabo LJ, Rouse M, Johnson J, et al. (2015) Phenotypic and genotypic characterization of race TKTTF of Puccinia graminis sp. triticithat caused a wheat stem rust epidemic in southern Ethiopia in 2013–14. Phytopathology 105: 917-928. [crossref]
- Abebe T, Woldeab G, Dawit W (2012) Distribution and Physiologic Races of Wheat Stem Rust in Tigray, Ethiopia. Journal of Plant Pathology and Microbiology 3: 142.
- Hailu A, Woldeab G, Dawit W, Hailu E (2015) Distribution of wheat stem rust (Puccinia Graminis F. Sp. Tritici) in West and Southwest Shewa Zones and identification of its physiological races. Advances in Crop Science and Technology 3: 189.
- Roelfs AP (1992) Rust diseases of wheat: concepts and methods of disease management. Cimmyt.
- Stakman EC, Stewart DM, Loegering WQ (1962) Identification of physiologic races of Puccinia graminis var. tritici. Washington: USDA 5-50.
- Tesfaye L (2016) Seedling resistance to stem rust (Puccinia graminissp. tritici) and molecular marker analysis of resistance genes in some wheat cultivars. plant 6: 16-23.
- Peterson RF, Campbell AB, Hannah AE (1948) A diagrammatic scale for estimating rust intensity of leaves and stem of cereals. Can J Res Sect C 26: 496-500.