Abstract
Chronic lung diseases like chronic obstructive pulmonary disease (COPD) and pulmonary fibrosis are progressive, life-limiting conditions that place a burden on both the patient and the healthcare system. Current treatments are aimed at improving symptoms but to date there are no treatments that can alter the natural course of disease. Innovative treatments for these diseases are needed urgently. Autologous cellular therapy shows promise for reducing chronic inflammation, modulating the immune system and promoting healing of tissue. This study is a retrospective review of quality of life scores over the year following autologous cellular therapy and shows both statistically and clinically significant improvement in patients with COPD and pulmonary fibrosis.
Keywords
COPD, pulmonary fibrosis, cellular therapy, platelet rich plasma
Introduction
Chronic lung diseases are a complex, global health problem and one of the leading causes of disability, morbidity and mortality worldwide [1]. There is increasing awareness that these diseases are multidimensional where the patient experiences not only physical symptoms but an array of psychosocial, behavioral and emotional stressors that can wax and wane between periods of exacerbations. Despite the known pulmonary and extra-pulmonary effects, patients with chronic lung disease are often marginalized by the health care system. In fact, chronic obstructive pulmonary disease (COPD) is listed in the bottom one-third of allocated funding for fiscal year 2019 by the National Institutes of Health [2condition, and disease categories based on grants, contracts, and other funding mechanisms used across the National Institutes of Health (NIH]. Despite available medications, many patients have exhausted the available pharmacologic treatment options and remain symptomatic.
Pharmacological treatments aim to improve the patient’s quality of life, manage symptoms and reduce flare-ups, though no medications have been conclusively shown to alter long-term decline in lung function [3]. Despite modern pharmacological science, chronic lung diseases remain an extreme burden, and novel treatments are needed urgently. Autologous cellular therapy, enhancing a patient’s intrinsic healing ability, is one innovative approach to treatment. This study examines the health-related quality of life outcomes of 2,813 COPD patients and 567 pulmonary fibrosis (PF) patients who underwent elective, autologous cellular therapy and shows both statistically and clinically significant improvements in post-treatment quality of life.
Background
Non-congenital lung diseases such as obstructive pulmonary disease (COPD) and pulmonary fibrosis (PF) are characterized by symptoms such as breathlessness, decreased activity tolerance and cough. Both COPD and PF are associated with chronic and irreversible airflow limitation and, in the case of PF, scarring of the lung parenchyma [4]. COPD tends to progress slowly whereas PF progresses more aggressively, with a median survival of just 3 years5lung disorders are a leading cause of morbidity and death worldwide. For many disease conditions no effective and curative treatment options are available. Cell therapies offer a novel therapeutic approach due to their inherent anti-inflammatory and anti-fibrotic properties. Mesenchymal stem/stromal cells (MSC. In the case of COPD, two main physiological processes occur: remodeling of the small airways and loss of alveolar attachments. Even in patients with mild COPD, there is detectable obstruction and airflow reduction in the peripheral airways [6]. Cigarette smoking is the most common cause of COPD though up to 30% of COPD patients have never smoked. Second-hand smoke, exposure to fumes and chemicals and air pollution have also been identified as causative agents [7].
Pulmonary fibrosis most often has no identifiable trigger [5lung disorders are a leading cause of morbidity and death worldwide. For many disease conditions no effective and curative treatment options are available. Cell therapies offer a novel therapeutic approach due to their inherent anti-inflammatory and anti-fibrotic properties. Mesenchymal stem/stromal cells (MSC]. Though some patients who develop PF are smokers, many have no attributable history of noxious exposure, contrasted with COPD patients who almost always have a history of cigarette smoking [6]. Cigarette smoke activates epithelial cells that produce inflammatory mediators, including TGF-B which promotes local fibrosis and tissue destruction. Growth factors such as VEGF, which are naturally occurring molecules able to stimulate cell growth and proliferation, are necessary for cell survival and integrity. In alveolar cells of the lungs, a reduction in VEGF has been found in smokers and patients with COPD [6]. Even after smoking cessation, a reduction in VEGF can continue, but the reason for this is not currently known6. In addition to inflammation, those with chronic lung disease experience an accelerated ageing of the lung tissue due to a defect in intrinsic anti-aging molecules. COPD shows prominent age-related features, including an increase in stem cell exhaustion, cellular senescence and a reduction in normally protective mechanisms such as cell , which allows for the methodical break-down and recycling of cellular components [8].
Cells, including those in the lungs, have a limited number of divisions in their lifetime. The ability of cells to maintain division comes from both intrinsic factors (such as telomere length) and extrinsic forces (such as oxidative stress). Once the cell’s DNA is too damaged from repeated imperfect, divisions, or the telomeres become exhausted, cells lose the potential to divide and become senescent. Because the processes of autophagy also becomes dysfunctional, senescent cells will stay metabolically active and can continue to alter their environment, releasing damaging biochemicals and pro-inflammatory mediators. At the cellular level, lungs affected by COPD and PF exhibit increased decreased cellular autophagy, increased cellular senescence, genomic instability, mitochondrial dysfunction, epigenetic changes and stem cell exhaustion [6,9].
In a young, healthy organism, cell senescence is a beneficial process that allows for the removal of potentially damaged and cancer-causing cells from the body. These damaged cells are replaced with an effective system of stem cells to re-establish cell numbers. As organisms age and chronic disease develops, the mobilization of progenitor stem cells decreases, and harmful senescent cells increase while there are no healthy replacements [9]. In addition, the normal mechanism of cell apoptosis, or the self-destruction and complete removal of non-functional cells may be further increased in COPD and PF allowing for additional dysfunctional cells to accumulate [9].
Almost all lung diseases involve some degree of inflammation and cellular dysfunction. Platelets, cells generally thought of as anucleate fragments of blood cells that regulate hemostasis, also play a crucial role in inflammation. For that reason, the role of platelets and their biology in the process of tissue repair and regeneration has been of interest in the past several years10,11. Aside from their importance in hemostasis, platelets and their secreted products function as circulating cell receptors that provide a an intricate link between immune responses and cellular repair [10]. Platelets contain an extraordinary number of biologically active growth factors that can be released with activation, including VEGF and PDGF, a growth factor which recruits immune cells. Platelets are important in vascular repair and maintaining integrity within the vast vasculature of the lungs [4]. Activated platelets release a wide range of factors that can also promote the recruitment, adherence and proliferation of stem cells, including CD-34 positive progenitor cells. CD-34-positive progenitor cells can increase vascular tissue repair by paracrine mechanisms (cell-to-cell communication where cells can influence the behavior of neighboring cells) [5lung disorders are a leading cause of morbidity and death worldwide. For many disease conditions no effective and curative treatment options are available. Cell therapies offer a novel therapeutic approach due to their inherent anti-inflammatory and anti-fibrotic properties. Mesenchymal stem/stromal cells (MSC]. Platelets can enhance the migration of adult stem cells to areas of cellular injury and can therefore be important in regenerative therapies. In addition, platelets play a role in antiapoptotic mechanisms, moving the balance from cell death to cell survival and restoration [4]. Though the exact mechanisms of tissue repair are not fully understood, there is a wide range of research showing that platelets are able to promote the release of beneficial cytokines, chemokines and growth factors and orchestrate the recruitment and activation of adult stem cells involved in healing [10].
Platelets can be isolated from whole blood and activated by a specialized centrifugation process. The process of concentrating platelets and their secreted molecules yields a product called platelet-rich plasma- platelet concentrate, or PRP-PC. The activation process releases multiple growth factors such as PGDF, VEGF, IGF-1 and HGF from the alpha granules of the platelets. For this study, the commercial preparation equipment used produces 6 times baseline platelet concentration, 3 to 5 times baseline white blood cell concentration and a reduction in red blood cells by 3 to 6 times baseline levels according to independent studies [12].
Measuring Treatment Response
Traditionally, the diagnosis of COPD has been made based on abnormal spirometry. PF can be diagnosed by computerized tomography (CT) scan, lung biopsy and pulmonary function testing that indicates abnormal diffusion of oxygen, but spirometry often appears normal. To measure the effectiveness of treatment, health care providers have used spirometry and, for COPD, measurement of the FEV1 (forced expiratory volume in 1 second). Although FEV1 decline has been correlated in studies with increased respiratory symptoms, it is only modestly associated with changes in quality of life and extrapulmonary effects of the disease [13,14COPD has both pulmonary and extrapulmonary effects, which have an impact on many aspects of physical, emotional, and mental well-being. Traditional assessment of COPD relies heavily on measuring lung function, specifically forced expiratory volume in 1 second (FEV(1]. Quality of life- the physical, emotional and social effects on the patient is not measured by spirometry. The term health-related quality of life has been defined as the ‘functional effect of an illness and its consequent therapy upon a patient, as perceived by the patient’ and therefore measurement of quality of life can only be reported reliably by the patient [13COPD has both pulmonary and extrapulmonary effects, which have an impact on many aspects of physical, emotional, and mental well-being. Traditional assessment of COPD relies heavily on measuring lung function, specifically forced expiratory volume in 1 second (FEV(1]. This study uses a self-report instrument, the Clinical COPD Questionnaire (CCQ) to evaluate the patient perspective. Health status questionnaires like the CCQ provide a comprehensive, measurable assessment of the overall patient experience of disease [13,15COPD has both pulmonary and extrapulmonary effects, which have an impact on many aspects of physical, emotional, and mental well-being. Traditional assessment of COPD relies heavily on measuring lung function, specifically forced expiratory volume in 1 second (FEV(1].
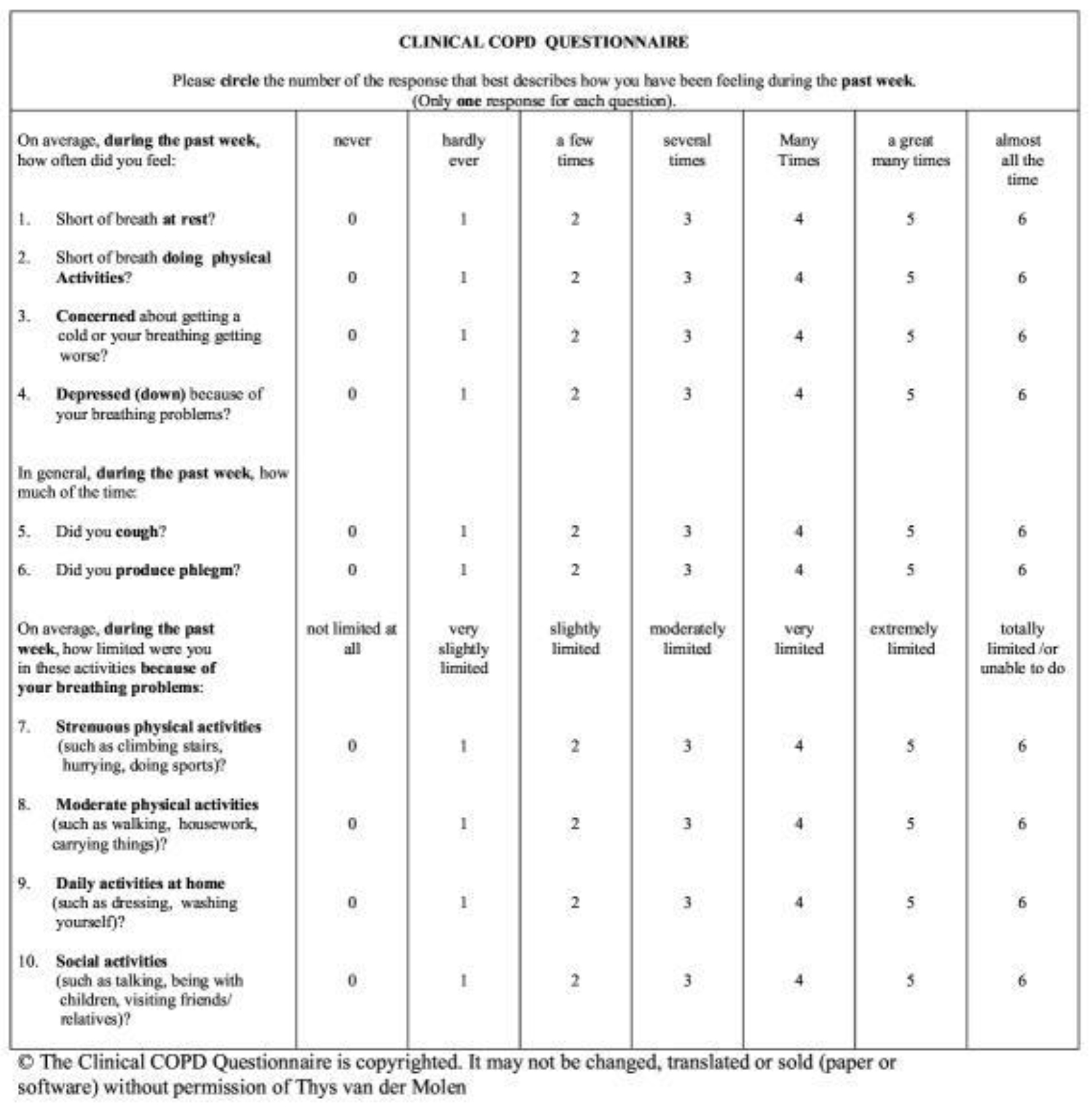
The CCQ has been widely used in a multitude of clinical settings. It is a short, ten-item tool developed in 2003 and translated into 64 different languages. It is not only useful for measuring baseline status but in measuring response to treatment [16many clinical trials now include assessments of PROs. These are formalized methods of capturing patient-centered information. Given the importance of PROs in evaluating the potential utility of an intervention for a patient with COPD, it is important that physicians are able to critically interpret (and critique]. Validation of the CCQ shows robust discriminative properties, good test-retest reliability and responsiveness. Although developed for use in COPD patients, it has also been tested and shown to be valid and reliable in other chronic lung diseases and even in patients at risk for COPD. Importantly, validation studies show that the CCQ is very sensitive for clinical changes after intervention [15]. The CCQ has three domains: the symptom domain (4 items), mental status domain (2 items) and functional status domain (4 items). Each of the ten items is ranked by the patient on a scale of 0 to 6, with 0 being not troublesome at all, to 6 being extremely troublesome. The final total score (0–60) is divided by ten to yield a final 0–6 score. In order to confirm that a change in score is clinically meaningful from baseline, Alma and associates have determined the minimum threshold of at least 0.4 points should be met [17,18].
Methods and Materials
All patients in this study underwent autologous harvest and isolation of platelet-rich plasma/platelet concentrate (PRP-PC) from peripheral blood. 120 mL of blood was collected from each patient over a period of 2 to 3 days. Each treatment day, whole blood was collected from the peripheral circulation, processed using centrifugation, and administered to the patient back through the peripheral circulation following approximately 15 minutes of centrifugation. As the cellular product (with a final yield of 8–10mL of PRP-PC) enters the peripheral vein, the natural circulatory system transports the product through the right heart and into the pulmonary circulation, where cells become trapped within the pulmonary microvasculature. Each patient was presented with informed consent prior to treatment. The treatment was very well-tolerated, and no adverse events were reported. Permission to evaluate the documented outcomes of patients treated was granted by an Institutional Review Board.
Before treatment, each patient was administered the CCQ. A copy of the CCQ is found in the illustrations. Patients were then contacted by phone by a licensed and trained research nurse at 3, 6 and 12-months post-treatment and the CCQ was re-administered and scored. To prevent confounding or bias, nurses did not have access to the previously answered CCQ. Responses to each question was recorded in an encrypted, online database for later data extraction and analysis. For this study, cases where a patient may not have answered all ten CCQ items were eliminated to prevent skewed data. Of course, over time, some subjects were lost to attrition, as evidenced by decreasing numbers of recorded patient outcomes at the 3, 6 and 12-month time points.
IBM’s Statistical Package for the Social Sciences (SPSS) was used to analyze the data points. Descriptives of the sample were run. Because of the discrete symptom, mental status and functional status domains and their importance as separate domains, total and average domain scores were also calculated and examined. Each data point was analyzed for a statistically significant change from baseline as well as a clinically significant change from baseline, indicated by a minimal clinically important difference (MCID) of 0.4 or more as per Alma, et al.
Results
The mean age of COPD-diagnosed patients was 70.8 years. 1,666 were male, 1,147 were female. The baseline CCQ among this group was 3.51 (N=2,813). At 3 months post-treatment the mean CCQ was 2.4 (N=2,008) and 1.1 improvement from baseline; at 6 months 2.42 (N=1,598) and 1.09 improvement from baseline and at 12 months 2.73 (N=754) 0.78 improvement from baseline which all far exceed the MCID of 0.4 T-tests show statistically significant improvement at all three time points from baseline with P < 0.001 for all.
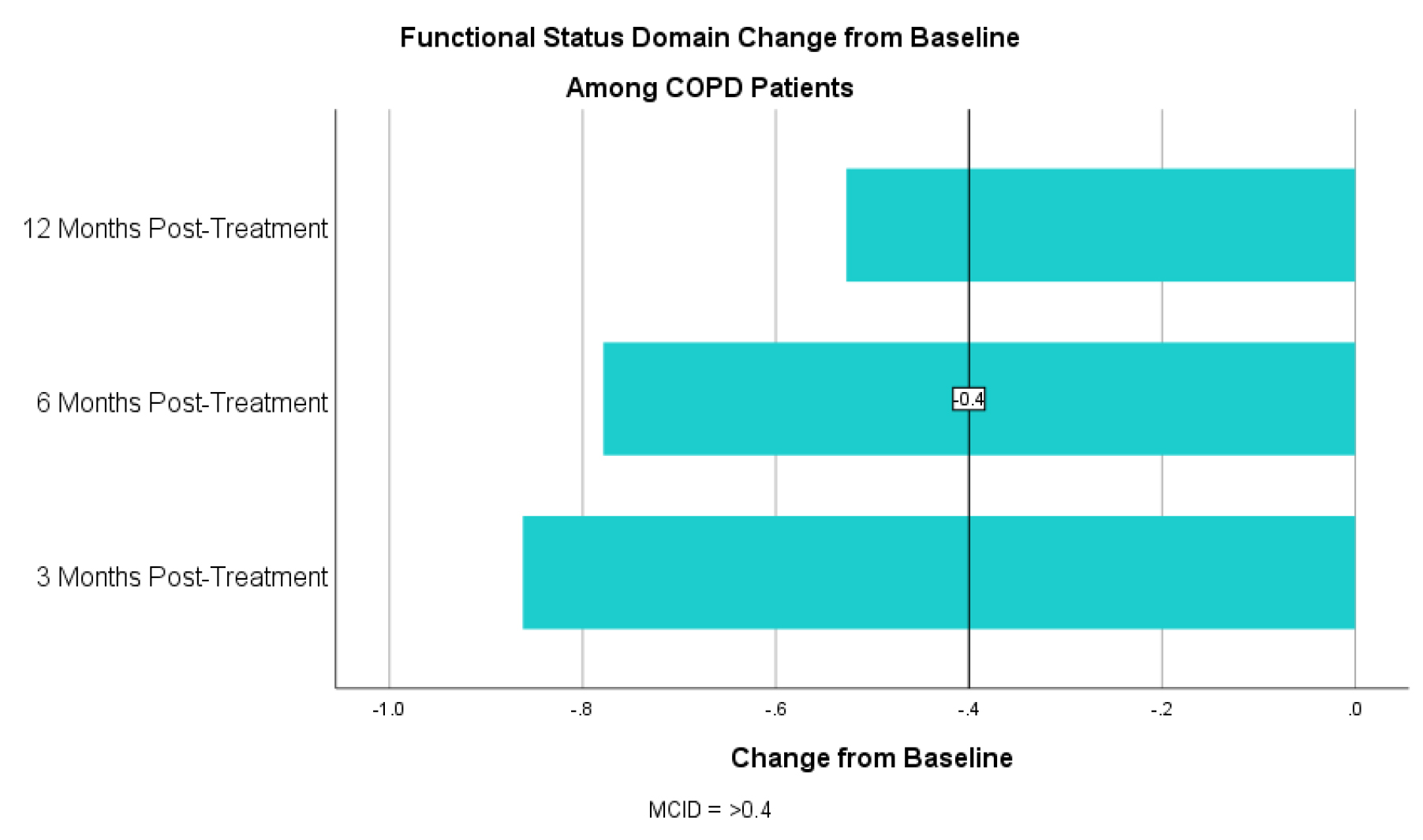
For the symptom domain, COPD patients had an improvement in CCQ by 1.16 at 3 months, 1.22 at 6 months and 0.93 at 12 months post-treatment (table 1). For the mental status domain there was an improvement in CCQ by 1.55 at 3 months, 1.49 at 6 months and 1.19 at 12 months (table 2). For the functional status domain there was an improvement in CCQ by 0.83 at 3 months, 0.77 at 6 months and 0.44 at 12 months (table 3). All exceed the MCID of 0.4 or more. T-tests show statistically significant improvement at all three time points and all three domains from baseline with a p < 0.01.
Table 1. CCQ Symptom Domain Change from Baseline Among COPD Patients
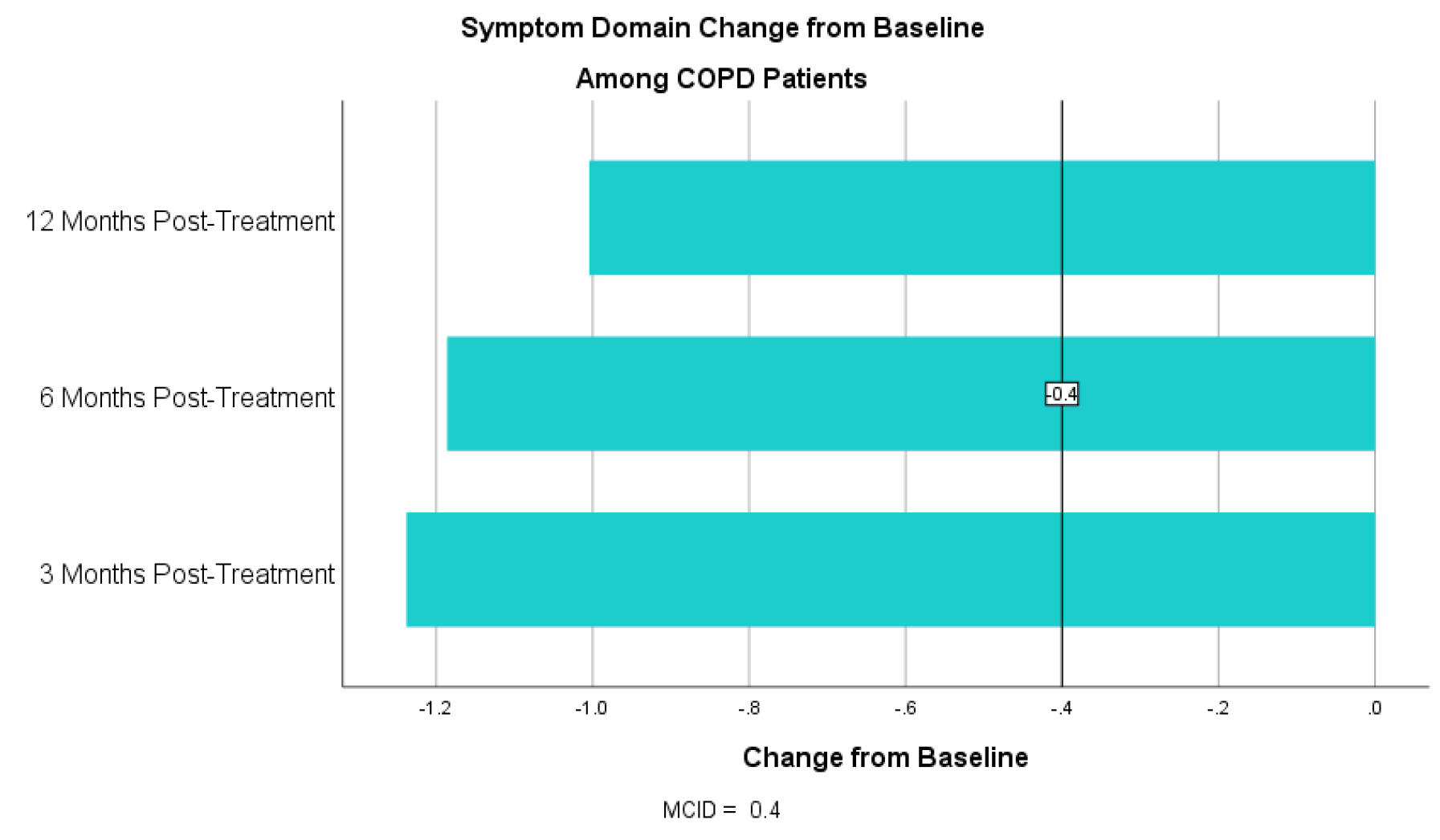
Table 2. CCQ Mental Status Domain Change from Baseline for COPD Patients
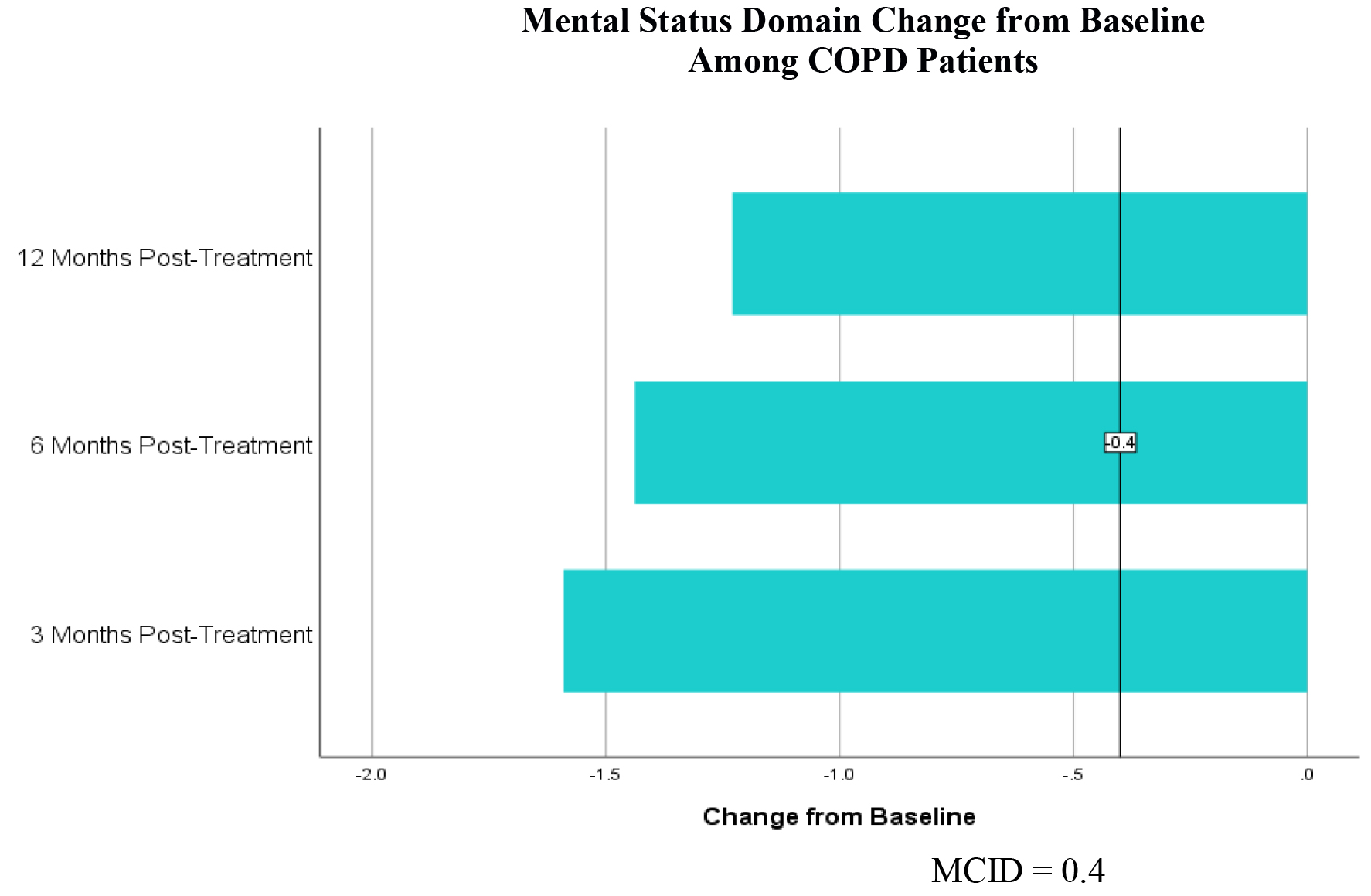
Table 3. CCQ Functional Status Domain Change from Baseline for COPD Patients
Concurrent with the MCID threshold of 0.4 or more, 75% (N=1,507) of COPD patients experienced improvement in quality of life at 3 months, 72.6% (N=1,161) experienced improvement in quality of life at 6 months and 64.1% (N=483) experienced improvement at 12 months post-treatment.
The mean age of patients with PF was 71.1 years. 389 patients were male, 178 were female. The baseline CCQ among this group was 3.34 (N=567). At 3 months, the mean CCQ was 2.53 (N=399) and a change of 0.81. At 6 months the mean CCQ was 2.69 (N=286) with a change of 0.65 and at 12 months the mean CCQ was 2.85 (N=124) with a change from baseline of 0.49. All time points exceed the MCID of 0.4. T-tests show statistically significant improvement at all three time points from baseline with a p <0.01 for all.
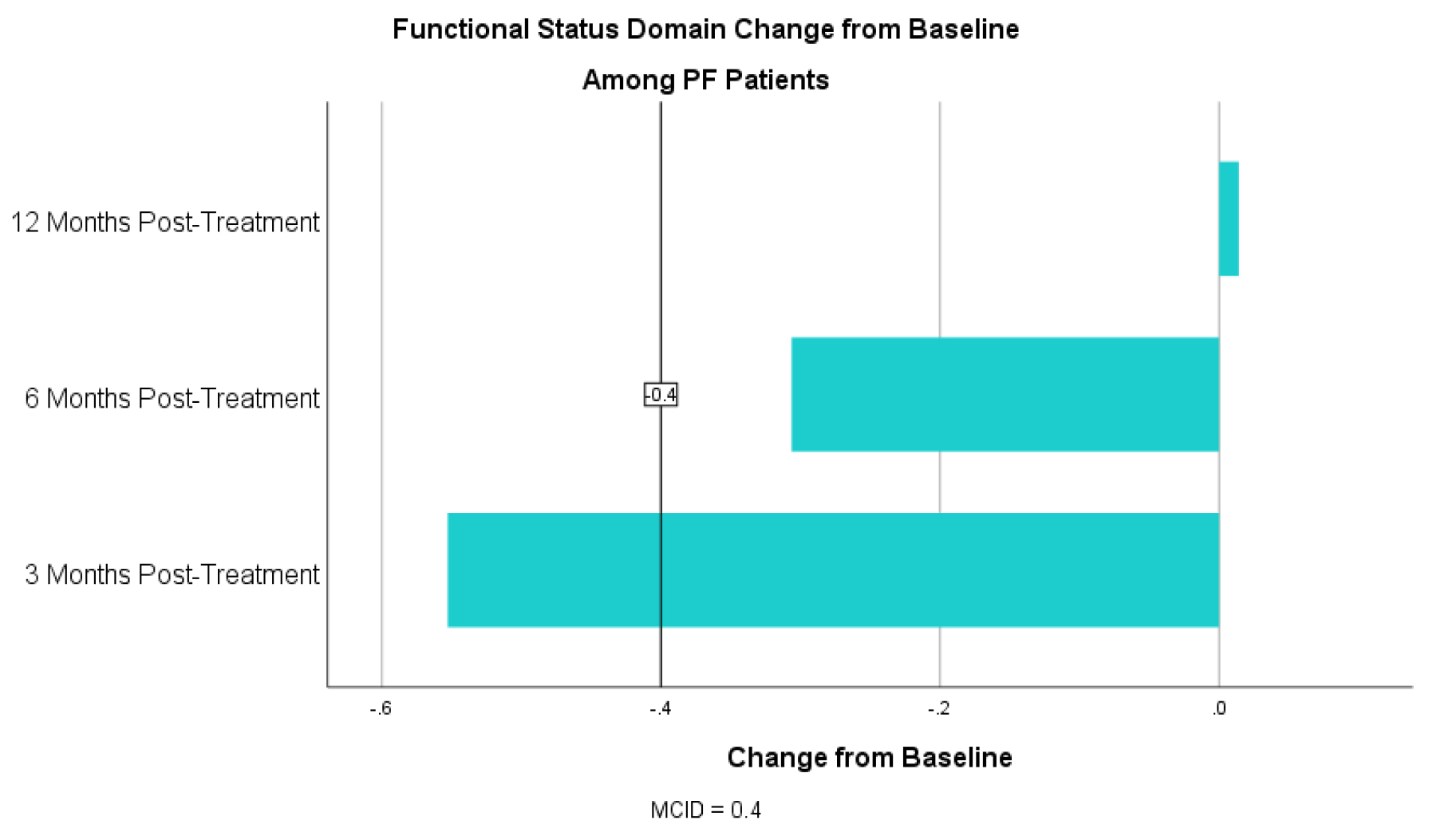
For the symptom domain, PF patients had an improvement in CCQ by 0.89 at 3 months, 0.77 at 6 months and 0.70 at 12 months (table 4). For the mental status domain there was an improvement in CCQ by 1.25 at 3 months, 1.07 at 6 months and 0.82 at 12 months (table 5). For the functional status domain there was an improvement in CCQ 0.52 at 3 months, 0.31 at 6 months and 0.10 at 12 months (table 6). The 6- and 12-month functional domain scores fell below the MCID of 0.4. T-tests, however, were statistically significant at for all domains at all time points with a p <0.01 for all (table 5).
Table 4. CCQ Symptom Domain Change from Baseline for PF Patients
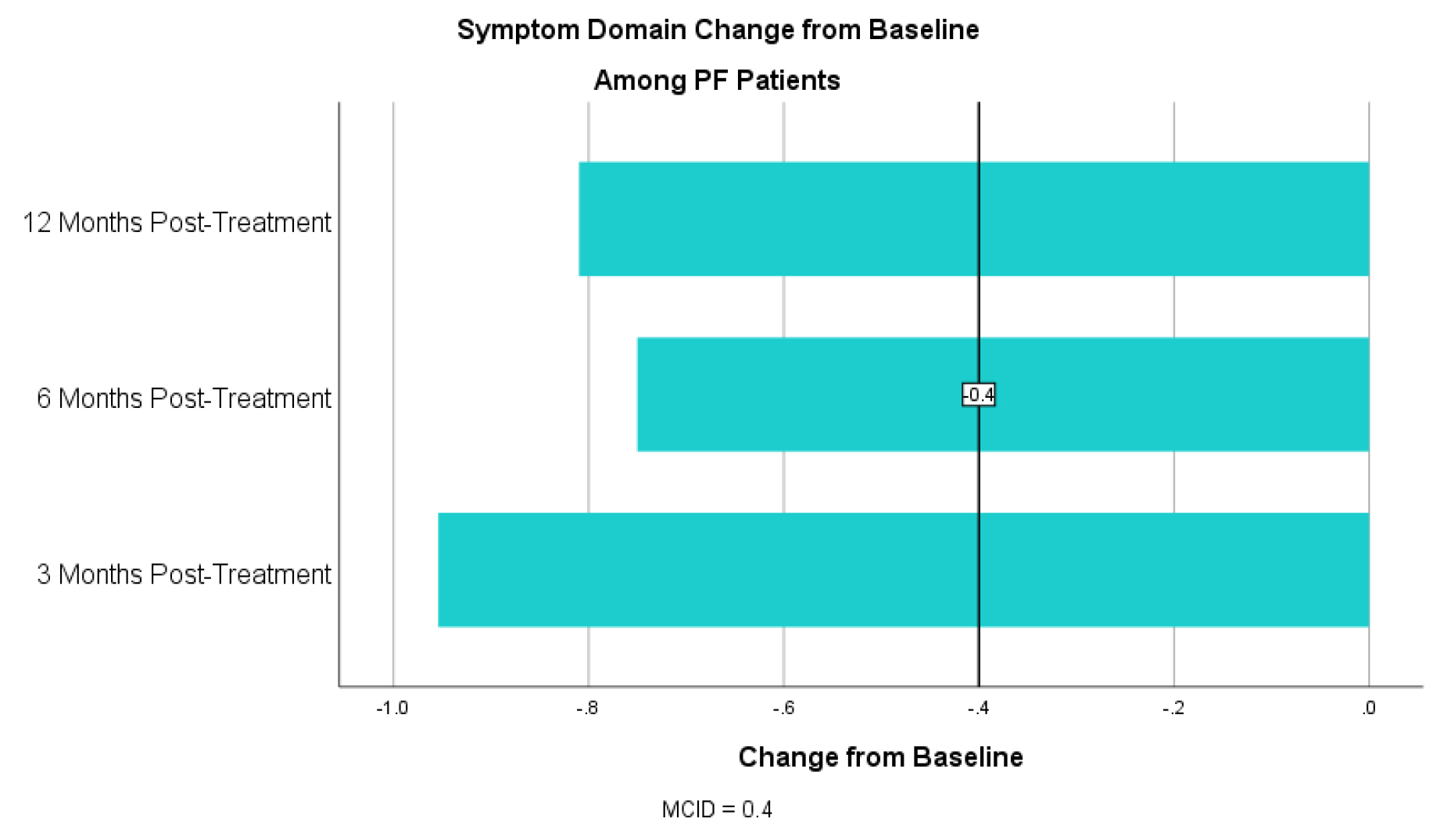
Table 5. CCQ Mental Status Domain Change from Baseline for PF Patients
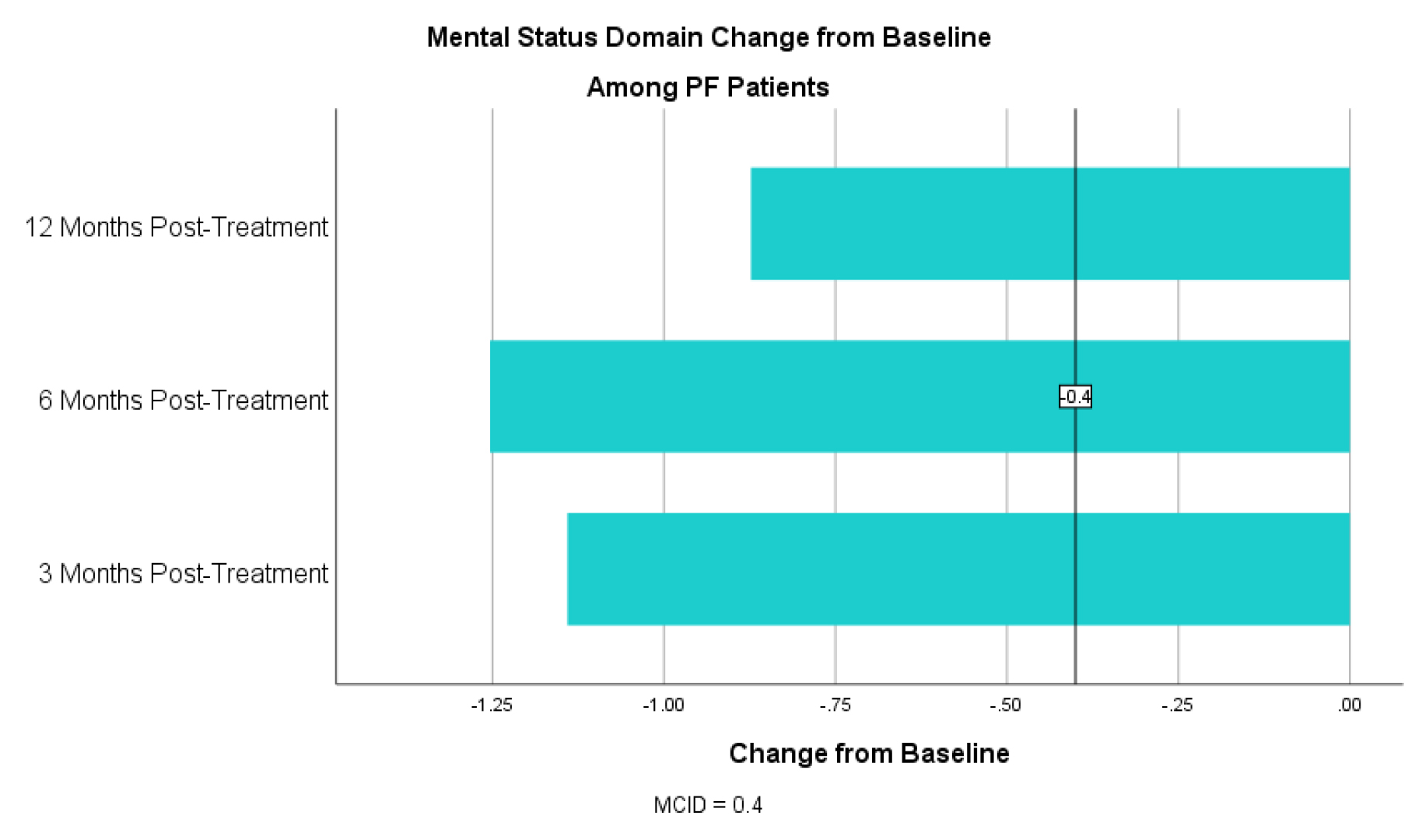
Concurrent with the MCID threshold of 0.4 or more, 66.2% (N=264) of PF patients experienced an improvement in quality of life at 3 months, 59.4% (N=170) experienced improvement at 6 months and 47.6% (N=59) experienced improvement at 12 months post-treatment (table 6).
Table 6. CCQ Functional Status Domain Change from Baseline for PF Patients
Discussion
There is a growing body of evidence that cellular therapies have great potential influence inflammatory, immune and regenerative functions in the lung. Although there is much yet to learn about the exact mechanisms of PRP-PC activity in lung tissue, this study shows both a statistically and highly clinically meaningful improvement in quality of life for patients with COPD and PF at nearly all post-treatment time points. COPD outcomes likely exceed PF outcomes here as PF is a faster-progressing disease with a mean survival of less than 5 years, though both groups have experienced quality of life improvement.
The symptom domain, which addresses breathlessness, coughing and phlegm production, and the mental status domain, which addresses symptoms of anxiety and depression related to breathing, have responded the best among this group. The functional domain, which addresses physical limitations due to disease, also shows overall improvement though to a lesser extent. These items, which account for heavy lifting and strenuous activity, are commonly difficult for those limited by chronic lung disease.
This study is limited to observation of outcomes and does not compare treatment with the standard pharmacologic treatments or placebo. All patients in this study were asked to continue their prescribed regimen during and after our treatment but this study cannot account for changes in treatment, new medical diagnoses or other alternative therapies. In addition, all patients were required to be non-smokers at the time of treatment, but this study does not account for those who may have resumed smoking in the year following treatment which may negatively affect outcomes.
Nonetheless, the outcomes presented here were tested for both clinical and statistical significance and, at almost all data points, far exceeds those tests, demonstrating that cellular therapy is a promising option for those who suffer with chronic lung diseases.
Acknowledgments
I would like to express my gratitude to Dr. Jack Coleman, Senior Medical Director at the
Lung Health Institute, for his knowledge and expertise in the arena of regenerative medicine. I would like to thank Shawn Clymer, data analyst, for collecting the data needed for this study.
Supporting Information
Clinical COPD Questionnaire
Abbreviations
|
CCQ |
Clinical COPD Questionnaire |
|
COPD |
Chronic obstructive pulmonary disease |
|
CT |
Computerized tomography |
|
FEV1 |
Forced expiratory volume in 1 second |
|
HGF |
Hepatocyte growth factor |
|
IGF-1 |
Insulin-like growth factor – 1 |
|
MCID |
Minimal clinically important difference |
|
PDGF |
Platelet-derived growth factor |
|
PF |
Pulmonary fibrosis |
|
PRP |
Platelet rich plasma |
|
PRP-PC |
Platelet rich plasma/platelet concentrate |
|
TGF-B |
Transforming growth factor beta |
|
VEGF |
Vascular endothelial growth factor |
Competing Interests
The author declares there are no competing interests.
Funding Information
No funding was used for this study.
References
- Koskela J, Kilpeläinen M, Kupiainen H, et al. (2014) Co-morbidities are the key nominators of the health related quality of life in mild and moderate COPD. BMC Pulm Med. doi: 10.1186/1471-2466-14-102.[Crossref]
- National Institute of Health. Estimates of Funding for Various Research, Condition, and Disease CAtegories (RCDC).; 2018.
- Yeh GY, Horwitz R. (2017) Integrative Medicine for Respiratory Conditions: Asthma and Chronic Obstructive Pulmonary Disease. Med Clin North Am. doi: 10.1016/j.mcna.2017.04.008. [Crossref]
- O’Sullivan BP, Kerrigan SW. (2015) Platelets, Inflammation and Respiratory Disease. In: The Non-Thrombotic Role of Platelets in Health and Disease. doi: 10.5772/60569.
- Geiger S, Hirsch D, Hermann FG. (2017) Cell therapy for lung disease. Eur Respir Rev. doi: 10.1183/16000617.0044–2017. [Crossref]
- Barnes PJ. (2016) Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. doi: 10.1016/j.jaci.2016.05.011. [Crossref]
- Lõpez-Campos JL, Tan W, Soriano JB. (2016) Global burden of COPD. Respirology. doi: 10.1111/resp.12660. [Crossref]
- Mercado N, Ito K, Barnes PJ. (2015) Accelerated ageing of the lung in COPD: New concepts. Thorax. doi: 10.1136/thoraxjnl-2014-206084. [Crossref]
- Mercado N, Ito K, Barnes PJ. (2015) Accelerated ageing of the lung in COPD: new concepts. Thorax. doi: 10.1136/thoraxjnl-2014-206084. [Crossref]
- Crisci A, Crescenzo U De, Crisci M. (2018) Platelet-rich concentrates (L-PRF, PRP) in tissue regeneration: Control of apoptosis and interactions with regenerative cells. J Clin Mol Med. doi: 10.15761/jcmm.1000116.
- Weyrich AS, Zimmerman GA. (2013) Platelets in Lung Biology. Annu Rev Physiol. (75): 569–591. [Crossref]
- Fitzpatrick J, Bulsara MK, McCrory PR, Richardson MD, Zheng MH. (2017) Analysis of Platelet-Rich Plasma Extraction: Variations in Platelet and Blood Components Between 4 Common Commercial Kits. Orthop J Sport Med. doi: 10.1177/2325967116675272. [Crossref]
- Jones P, Miravitlles M, van der Molen T, Kulich K. (2012) Beyond FEV1 in COPD: A review of patient-reported outcomes and their measurement. Int J COPD. 7: 697–709. doi: 10.2147/COPD.S32675.
- Tantucci C, Modina D. (2012) Lung function decline in COPD. Int J Chron Obstruct Pulmon Dis. 7: 95–99. doi: 10.2147/COPD.S27480. [Crossref]
- Kocks JWH, Tuinenga MG, Uil SM, van den Berg JWK, Ståhl E, van der Molen T. (2006) Health status measurement in COPD: the minimal clinically important difference of the clinical COPD questionnaire. Respir Res. 7(i): 62. [Crossref]
- Jones PW, Rennard S, Tabberer M, Riley JH, Vahdati-Bolouri M, Barnes NC. (2016) Interpreting patient-reported outcomes from clinical trials in COPD: A discussion. Int J COPD. doi: 10.2147/COPD.S117378. [Crossref]
- Alma H, De Jong C, Jelusic D, et al. (2016) Health status instruments for patients with COPD in pulmonary rehabilitation: Defining a minimal clinically important difference. npj Prim Care Respir Med. 26. doi: 10.1038/npjpcrm.2016.41. [Crossref]
- Alma HJ, de Jong C, Jelusic D, et al. (2018) Assessing health status over time: impact of recall period and anchor question on the minimal clinically important difference of copd health status tools. Health Qual Life Outcomes. doi: 10.1186/s12955-018-0950-7. [Crossref]