Abstract
Goals: This study is aimed to analyze the results of external audits in medical facilities in Russia.
Design: Analysis of the results of audits in terms of the sections: “Epidemiologic safety. Preventing and Controlling Healthcare Associated Infections”, “Drug safety. Pharmacovigilance”, “Control of quality and safety of medical devices circulation”, and “Surgical safety. Preventions of risks associated with surgical intervention” in medical facilities of Russia.
Setting: 10 medical facilities in which the quality management system had not been implemented before.
Results: Nowadays the absence of unified approaches to the management of quality and safety of medical care is one of the most complicated and debatable issues in the medical society in Russia. Within the framework of this study, we have analyzed the results of external audits of quality and safety of medical care conducted in accordance with several sections of the Guidelines in medical facilities in which the quality management system had not been implemented before. The lowest level of conformity (15,9%) was found for the “Epidemiologic safety. Preventing and Controlling Healthcare Associated Infections”. The organizational problems were found in medical devices circulation, microbiologic monitoring systems, and systems of registration and collection of information concerning severe and unexpected adverse drug reactions. The practice of audits in medical facilities revealed essential structural problems with medical care quality and safety management in Russia.
Key words
medical care, Russia, audit, quality and safety, quality management system
Introduction
Nowadays the absence of unified approaches to the management of quality and safety of medical care is one of the most complicated and debatable issues in the medical society. There is no developed unified regulatory standard for management of medical care in medical facilities in Russia now.
In order to solve this problem, in 2015 Federal State Budgetary Institution “Center for Monitoring and Clinical and Economic Expert Evaluation” of Federal Service for Surveillance in Healthcare developed Roszdravnadzor’s Practical Guidelines (Recommendations) on the internal system of quality and safety control of medical care in medical facilities [1]. These Guidelines became the prototype of the national safety and quality healthcare standard for hospitals in Russia. The Guidelines were developed with due consideration of the requirements of current worlds standards: Joint Commission International Standards for Hospital (USA), National Safety and Quality Health Service Standards (Australia), Canadian Council on Health Services Accreditation (Canada), and others.
The Guidelines provided the basis for the System of the voluntary certification of medical facilities “Quality and Safety of Medical Care”, which was registered in 2016 [3].
Audit is the form of evaluation of the conformity of the medical facility to the requirements of the Guidelines [4]. Audits are to be carried out by specialists from a separate independent organization who are experts in the field.
This system implies external evaluations (audits) of medical facilities regarding the compliance with the requirements of the Guidelines.
The Guidelines include the following main fields of concern:
- Human resources management;
- Patient Identification;
- Epidemiologic safety. Preventing and Controlling Healthcare Associated Infections;
- Drug safety. Pharmacovigilance;
- Control of quality and safety of medical devices circulation;
- Emergency care in inpatient facilities;
- Managing clinical responsibility. Patient internal and external transfer;
- Surgical safety. Preventions of risks associated with surgical intervention;
- Blood management;
- Safe environment for the delivery of care. Patient care management. Preventing and managing falls, pressure injuries;
Methods
In this article, we will analyze the results of external audits of quality and safety of medical care conducted in accordance with several sections of the Guidelines in medical facilities in which the quality management system had not been implemented before. The audits were carried out by multidisciplinary work groups of experts under the supervision of experts from the Federal State Budgetary Institution “Center for Monitoring and Clinical and Economic Expert Evaluation” of Federal Service for Surveillance in Healthcare by the unified procedure based on the Guidelines.
Within the framework of this study, we have analyzed the results of audits carried out in terms of the following sections: “Epidemiologic safety. Preventing and Controlling Healthcare Associated Infections”, “Drug safety. Pharmacovigilance”, “Control of quality and safety of medical devices circulation”, and “Surgical safety. Preventions of risks associated with surgical intervention”.
The assessment sheet for these sections includes the list of criteria combined into groups. The assessment system is binary; it determines the conformity or non-conformity to one or another criterion. The non-conformity to any criterion in the group is the reason to consider the whole group of parameters non-conforming. For example, when evaluating the requirement concerning the availability of the microbiology testing system the experts checked the conformity to the following criteria: availability of an own microbiology laboratory or an agreement, necessary conditions for material sampling 24 hours per day, 7 days per week, 365 days per year, including the availability of transport media, thermostats and procedures of material sampling for all possible cases for a certain medical facility, personnel’s knowledge of procedures (interviewing) and practical skills (observation), and following the procedures, which was assessed using the method of studying medical records, and the criteria of timeliness of getting the results of cultures and their proper use for changing the empiric regimen of antimicrobial therapy for another regimen taking into account the sensitivity. Only positive answers to all the questions provided a positive result of evaluation according to one (!) requirement.
The sources of information described on the Figure 1.

Figure 1. Sources of information
The article uses the results of external audits of medical facilities, which are super specialty hospitals that deliver both elective and emergency care including high-tech medical care. The average hospital bed capacity was 500 beds (up to 1000); the average number of the personnel (both healthcare professionals and allied health personnel) was about 2000 people in each facility. The main criterion for choosing a medical facility for this study was the fact that there was not the quality management system based on the ISO 9000 standards.
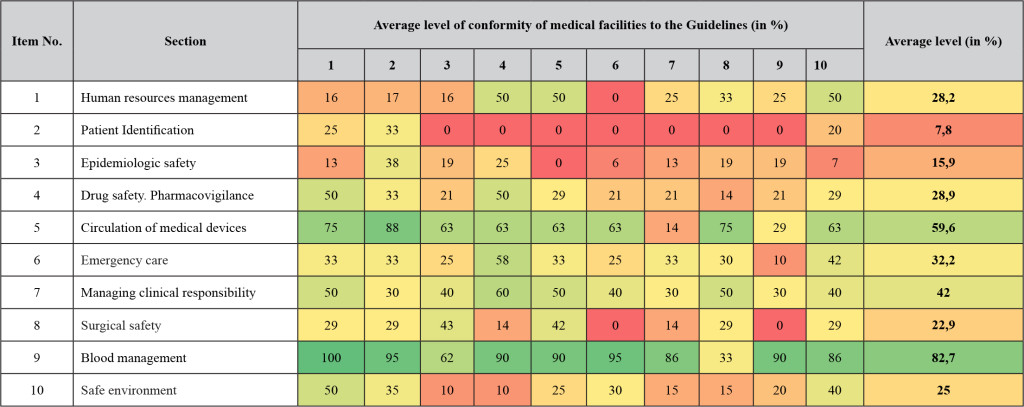
The summarized results of audits are shown in the Table 1.
Table 1. summarized results of audits
Initiators of conducting audits were the authorities of medical facilities. All the members of expert teams adhered to the principles of privacy and goodwill. Experts made a point of the fact that the authorities of the medical facilities in question had ensured the personnel that they would not be punished after the audit in any case which made the personnel more open. According to the conditions of the agreement between medical facilities and Federal State Budgetary Institution “Center for Monitoring and Clinical and Economic Expert Evaluation” of Federal Service for Surveillance in Healthcare the experts had access to all the rooms of the facilities and to all medical and organizational records.
Results
As it is shown in Table 1, the lowest level of conformity (15,9%) was found for the “Epidemiologic safety. Preventing and Controlling Healthcare Associated Infections” section. Almost all medical facilities in question had no effective microbiologic monitoring systems, no microbiology studies, and prudent use of antimicrobial drugs was not provided there. It is also necessary to make profound changes in revealing, recording, registration and analysis of infections associated with healthcare delivery.
When evaluating the “Surgical safety” section (the level of conformity – 22,9%), experts found organizational problems. For example, all the medical facilities in question have no functional surgical safety management system: a surgical check-list, procedures of transferring clinical responsibility in a post-surgery period, evaluation of anesthesia and pain management effectiveness in a post-surgery period were not developed and are not used. Many facilities do not use pain assessment scales which help to customize approaches to pain management.
The “Drug safety” section has the level of conformity equal to 28,9%. All the medical facilities in question have no effective systems of registration and collection of information concerning severe and unexpected adverse drug reactions. The labeling of vials with infusion solutions did not conform to the established criteria. There are still unsolved problems with the knowledge of procedures and the quality of verbal drug administration. Moreover, it is almost impossible to assess the conformity of the drug selection and dosage to clinical recommendations (treatment protocols) as there are no such recommendations at most workplace, though they can be found in the federal electronic medical library.
The procedure for medical devices circulation is assessed in the “Circulation of medical devices” section and has the level of conformity equal to 59,6%. Organizational problems with medical devices circulation were revealed during audits. In 9 of the 10 investigational medical facilities the system of medical devices circulation quality and safety monitoring is fragmentary, and a system approach is not used properly. The personnel are not being trained regarding issues of medical devices circulation quality and safety, and no internal audits are conducted. The personnel of medical facilities do not work properly with instruction manuals of medical devices. The requirements for correct use, maintenance, storage and disposal stated by manufacturers are just partially adhered to in all the assessed medical facilities.
Conclusion
The practice of conducting audits in medical facilities, where the quality management system had not been implemented before, revealed essential structural problems with medical care quality and safety management. The approach to the assessment of the medical care quality described in the Guidelines gives the opportunity to assess a medical facility in an integrated manner.
In contrast with the approved Russian practice of evaluating mainly submitted documents, the assessment in accordance with the Guidelines helps to reveal system problems based on several sources of information (profound observation over the processes of medical care, interviewing the personnel and patients). The evaluation process is more successful when the expert is maximally immersed in the clinical environment instead of documents.
The importance of studying the issues of epidemiologic, drug and surgical safety, the issues of medical devices circulation, the importance of management of risks associated with these spheres of medical care have met with support of the medical personnel and are regarded promising in Russia.
The further use of the Guidelines will help to improve approaches to solving these problems in order to change the situation for the better.
References
- Proposals for arrangement of inner quality and safety control of medical activity in medical organization (hospital) // Bulletin of Roszdravnadzor. — 2016. — N 2. — P. 35, 36.
- Ivanov I.V., Shvabskii O.R., Minulin I.B., Shchesyul A.G. Medical activity: quality and safety // Standards and Quality. – 2017. – N 3. – P.72–74.
- Ivanov I.V., Shvabskii O.R., Minulin I.B., Emanuel A.V. Audit as a tool of healthcare quality assessment // Standards and Quality. – 2017. – N 11. – P.27–29.
- Ivanov I.V., Shvabskii O.R., Minulin I.B., Shcheblykina A.A. Results of audits of quality and safety of medical activity in hospital. // Quality Management in Healthcare. – 2018. – N 1. – P. 18–22.